Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc - PubMed (original) (raw)
Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc
L Genestier et al. J Exp Med. 1999.
Abstract
Activation-induced cell death (AICD) is a process that regulates the size and the duration of the primary immune T cell response. In this report, we investigated the mechanisms involved in the regulation of AICD by transforming growth factor beta1 (TGF-beta1). We found that TGF-beta1 decreased apoptosis of human T cells or T cell hybridomas after activation by anti-CD3. This decrease was associated with inhibition of Fas (Apo-1/CD95) ligand (FasL) expression, whereas Fas signaling was not affected by TGF-beta1. In parallel, TGF-beta1 inhibited c-Myc expression in T cell hybridomas, and ectopic expression of a chimeric molecule composed of c-Myc and the steroid binding domain of the estrogen receptor (Myc-ER) blocked both the inhibition of FasL and the decrease of AICD induced by TGF-beta1, providing that 4-hydroxytamoxifen was present. These results identify one mechanism by which TGF-beta1 blocks AICD to allow the clonal expansion of effector T cells and the generation of memory T cells during immune responses.
Figures
Figure 1
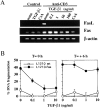
TGF-β1 decreases AICD in human peripheral blood T cells and murine T cell hybridomas. (A) PBMCs were activated for 6 d with OKT3 (100 ng/ml) and then restimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) (P+I) for 16 h with the indicated concentration of TGF-β1 or CsA (100 ng/ml). Viability was assessed by propidium iodide uptake and analyzed using a FACScan®. Apoptosis was confirmed by morphological assessment after staining with Hoechst 33342 at 10 μg/ml (not shown). (B) A1.1 or 2B4.11 T hybridoma cells were left unactivated (open symbols) or were activated (filled symbols) for 16 h with anti-CD3 antibody (2C11) in the presence of the indicated concentration of TGF-β1. Viability was assessed as in A. (C) A1.1 T hybridoma cells were cultured with medium alone or activated for 12 h with anti-CD3 antibody in the presence of the indicated concentrations of TGF-β1 (ng/ml) or CsA (100 ng/ml). DNA fragmentation associated with apoptosis was assessed by agarose gel electrophoresis.
Figure 2
TGF-β1 inhibits activation-induced apoptosis by blocking FasL mRNA expression and functional activity. (A) TGF-β1 inhibits activation-induced FasL mRNA expression. RT-PCR analysis of total mRNA obtained from A1.1 cells incubated in medium alone or activated for 4 h by anti-CD3 antibodies in the presence or absence of increasing concentrations of TGF-β1 or CsA (100 ng/ml). mRNA was reverse transcribed by using oligo(dT) primers, and PCR amplification was performed using different numbers of cycles with the primers pairs indicated. The products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide. (B) TGF-β1 inhibits AICD by modulating the expression of FasL functional activity but not by blocking the signaling through Fas receptor. A1.1 cells were activated at time 0 with coated anti-CD3 antibodies in the presence or absence of different concentrations of TGF-β1. The L1210 or L1210-Fas target cells were not added at this time. The T cell hybridomas were then cultured for 6 h to allow FasL expression, harvested, washed twice, and incubated for an additional 8 h with [3H]TdR-labeled L1210 or L1210-Fas target cells. In a parallel experiment, A1.1 cells were activated with anti-CD3 alone and TGF-β1 was added when A1.1 and target cells were mixed (T = +6 h). Percentage of DNA fragmentation was calculated as described in Materials and Methods.
Figure 3
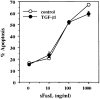
TGF-β1 does not block recombinant soluble FasL– induced apoptosis. A1.1 T cell hybridomas were preincubated for 1 h in the presence of TGF-β1 (10 ng/ml) and then treated with different doses of soluble recombinant human FasL (sFasL). 15 min after addition of soluble FasL, anti-Flag M2 antibody (1 μg/ml) was added to cross-link soluble FasL. After 12 h incubation, percentage of apoptosis was assessed by propidium iodide uptake and analyzed using a FACScan®.
Figure 4
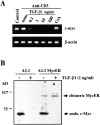
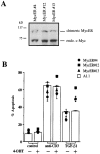
TGF-β1 inhibits endogenous c-myc mRNA and protein expression but not ectopic expression of the chimeric Myc-ER protein. (A) TGF-β1 inhibits c-myc mRNA expression. RT-PCR analysis of total mRNA obtained from A1.1 cells incubated in medium alone or activated for 4 h by anti-CD3 antibody with the indicated concentration of TGF-β1 or CsA (100 ng/ml). mRNA was reverse transcribed by using oligo(dT) primers, and PCR amplification was performed using different numbers of cycles with the primers pairs indicated. The products were resolved by agarose gel electrophoresis. (B) TGF-β1 inhibits constitutive endogenous c-Myc protein expression but not ectopic expression of the chimeric Myc-ER protein. Total cell extracts were prepared from A1.1 or A1.1 Myc-ER cells treated for 8 h in the presence or absence of TGF-β1 (1 ng/ml). Samples were analyzed by immunoblot analysis using anti–human c-Myc antibody.
Figure 5
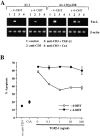
Ectopic expression of chimeric Myc-ER protein prevents TGF-β1–mediated inhibition of FasL mRNA and subsequent AICD. (A) Functional Myc-ER interferes with the inhibitory effect of TGF-β1 on activation-induced FasL expression in A1.1 cells. A1.1 or A1.1 Myc-ER cells exposed to 4-OHT (50 nM) were first preincubated for 4 h with the drug, which was then also present during subsequent culture. Cells were then incubated in medium alone (lane 1) or activated for 4 h with anti-CD3 antibodies (lanes 2, 3, and 4). 1 ng/ml TGF-β1 (lane 3) or 100 ng/ ml CsA (lane 4) was added to some cultures. FasL expression was then assessed by RT-PCR. (B) Ectopic expression of a chimeric Myc-ER protein prevents AICD after TGF-β1 treatment. A1.1 or A1.1 Myc-ER T hybridoma cells were first preincubated for 4 h in the presence or absence of 4-OHT (50 nM) and then activated for 16 h with anti-CD3 antibodies with the indicated concentrations of TGF-β1 (1 ng/ml) or CsA (100 ng/ml). Cell death was assessed by propidium iodide uptake using a FACScan®.
Figure 6
Ectopic expression of chimeric Myc-ER protein prevents TGF-β1–mediated inhibition of AICD in A1.1 T cell hybridomas. (A) Expression of Myc-ER fusion protein in A1.1 Myc-ER clones. Total cell extracts were prepared from three different A1.1 Myc-ER clones. Samples were analyzed by immunoblot analysis using anti–human c-Myc antibody. (B) Ectopic expression of a chimeric Myc-ER protein prevents AICD after TGF-β1 treatment. A1.1 or A1.1 Myc-ER clones were activated for 16 h with anti-CD3 antibodies with the indicated concentrations of TGF-β1 (1 ng/ml) in the presence or absence of 4-OHT (50 nM) added at time 0 of the activation. Cell death was assessed by propidium iodide uptake using a FACScan®.
Figure 7
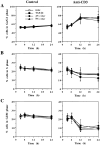
TGF-β1 as well as AS_c-myc_ oligonucleotides do not induce perturbation of the cell cycle in A1.1 T cell hybridomas. 106 A1.1 cells were untreated or activated with anti-CD3 in the presence of 5 μM AS_c-myc_ or NS_c-myc_ oligonucleotides added 4 h before activation or with TGF-β1 (10 ng/ml) added 1 h before activation. Cells were harvested at the indicated times, and cell cycle analysis was performed by propidium iodide staining after permeabilization with Triton X-100. The percentage of cells in G0/G1 (A), S (B), and G2/M (C) phase of the cell cycle was determined for each condition (data shown are means ± SD, n = 3).
Similar articles
- Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells.
Wang R, Zhang L, Zhang X, Moreno J, Celluzzi C, Tondravi M, Shi Y. Wang R, et al. Eur J Immunol. 2002 Apr;32(4):1090-8. doi: 10.1002/1521-4141(200204)32:4<1090::AID-IMMU1090>3.0.CO;2-P. Eur J Immunol. 2002. PMID: 11920576 - Fungal metabolite FR901228 inhibits c-Myc and Fas ligand expression.
Wang R, Brunner T, Zhang L, Shi Y. Wang R, et al. Oncogene. 1998 Sep 24;17(12):1503-8. doi: 10.1038/sj.onc.1202059. Oncogene. 1998. PMID: 9794227 - VIP and PACAP inhibit activation induced apoptosis in T lymphocytes.
Delgado M, Ganea D. Delgado M, et al. Ann N Y Acad Sci. 2000;921:55-67. doi: 10.1111/j.1749-6632.2000.tb06951.x. Ann N Y Acad Sci. 2000. PMID: 11193880 - Negative regulation of CD95 ligand gene expression by vitamin D3 in T lymphocytes.
Cippitelli M, Fionda C, Di Bona D, Di Rosa F, Lupo A, Piccoli M, Frati L, Santoni A. Cippitelli M, et al. J Immunol. 2002 Feb 1;168(3):1154-66. doi: 10.4049/jimmunol.168.3.1154. J Immunol. 2002. PMID: 11801650 - Promotion and inhibition of activation-induced apoptosis in T-cell hybridomas by oncogenes and related signals.
Green DR, Mahboubi A, Nishioka W, Oja S, Echeverri F, Shi Y, Glynn J, Yang Y, Ashwell J, Bissonnette R. Green DR, et al. Immunol Rev. 1994 Dec;142:321-42. doi: 10.1111/j.1600-065x.1994.tb00895.x. Immunol Rev. 1994. PMID: 7698799 Review.
Cited by
- T cell death and transforming growth factor beta1.
Golstein P, Wyllie AH. Golstein P, et al. J Exp Med. 2001 Aug 20;194(4):F19-22. doi: 10.1084/jem.194.4.f19. J Exp Med. 2001. PMID: 11514612 Free PMC article. No abstract available. - The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases.
Horwitz DA, Gray JD, Zheng SG. Horwitz DA, et al. Arthritis Res. 2002;4(4):241-6. doi: 10.1186/ar414. Epub 2002 Mar 12. Arthritis Res. 2002. PMID: 12106494 Free PMC article. Review. - Many checkpoints on the road to cell death: regulation of Fas-FasL interactions and Fas signaling in peripheral immune responses.
Ramaswamy M, Cleland SY, Cruz AC, Siegel RM. Ramaswamy M, et al. Results Probl Cell Differ. 2009;49:17-47. doi: 10.1007/400_2008_24. Results Probl Cell Differ. 2009. PMID: 19132321 Free PMC article. Review. - Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses.
McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. McNally JM, et al. J Virol. 2001 Jul;75(13):5965-76. doi: 10.1128/JVI.75.13.5965-5976.2001. J Virol. 2001. PMID: 11390598 Free PMC article. - Therapeutic targeting of TGF-β in cancer: hacking a master switch of immune suppression.
van den Bulk J, de Miranda NFCC, Ten Dijke P. van den Bulk J, et al. Clin Sci (Lond). 2021 Jan 15;135(1):35-52. doi: 10.1042/CS20201236. Clin Sci (Lond). 2021. PMID: 33399850 Free PMC article. Review.
References
- Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. - PubMed
- Kasid A, Bell GI, Director EP. Effects of transforming growth factor-beta on human lymphokine-activated killer cell precursors. Autocrine inhibition of cellular proliferation and differentiation to immune killer cells. J Immunol. 1988;141:690–698. - PubMed
- Fontana A, Frei K, Bodmer S, Hofer E, Schreier MH, Palladino MA, Jr, Zinkernagel RM. Transforming growth factor-beta inhibits the generation of cytotoxic T cells in virus-infected mice. J Immunol. 1989;143:3230–3244. - PubMed
- Bright JJ, Kerr LD, Sriram S. TGF-beta inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J Immunol. 1997;159:175–183. - PubMed
- Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous