Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population - PubMed (original) (raw)
Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population
F Agenès et al. J Exp Med. 1999.
Abstract
We studied the role of bone marrow B cell production in the renewal of peripheral B cells and the feedback mechanisms that control the entry of newly formed B cells into the peripheral B cell pools. When resting lymph node B cells are injected into B cell-deficient hosts, a fraction of the transferred cells expands and constitutes a highly selected population that survives for prolonged periods of time by continuous cell renewal at the periphery. Although the number of donor B cells recovered is low, a significant fraction shows an activated phenotype, and the serum immunoglobulin (Ig)M levels are as in normal mice. This population of activated B cells is resistant to replacement by a new cohort of B cells and is able to feedback regulate both the entry of newly formed B cells into the peripheral pool and terminal differentiation. These findings suggest that peripheral B cell selection follows the first come, first served rule and that IgM-secreting cells are generated from a pool of stable activated B cells with an independent homeostasis.
Figures
Figure 1
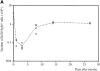
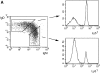
(A) Fate of LN B cells transferred into immunodeficient Rag2−/− mice. Each host mouse was injected with 107 cells (4 × 106 B cells). Each point represents the number of donor LN-derived B cells (B220+ μ+) recovered in the SPL of three to six host mice as a function of time. The curve joins the mean of the values obtained at each time point. (B) Phenotype of in vivo activated B cells. Rag2−/− mice were injected with 4 × 107 LN cells (1.8 × 107 B cells). The hosts were killed 2 mo later and the SPL cells analyzed by flow cytometry. Histograms are gated for the B220+ B cell population. Bold line, phenotypic analysis of LN-derived B cells. Thin line, phenotypic analysis of splenic B cells from C57Bl/6 control mice. (C) Contour plot showing the sorted B2 cells from CD3ε−/− donors before and after in vivo activation in Rag2−/− hosts. Similar observations were made when LN cells from the same donors were transferred. (D) Cytokine profile of in vivo activated B cells. RT-PCR was performed on sorted B cells (>99% pure). Lanes from the left to the right: splenic cells from Rag2−/− mice injected 1 mo before with LN cells from CD3ε−/− donors (pool of 10 mice); mature B cells (IgMlowIgDhigh) from the SPL of CD3ε−/− mice (pool of 5 mice); activated B cells (IgMhighIgDlow) from the SPL of CD3ε−/− mice (pool of 5 mice); B cells from the LN of CD3ε−/− mice (pool of 5 mice). (E) Number of IgM- secreting cells in the SPL of immunodeficient hosts injected with 107 cells (4 × 106 B cells) as a function of time. Each point represents one individual mouse and the curve joins the mean values obtained in each time point.
Figure 1
(A) Fate of LN B cells transferred into immunodeficient Rag2−/− mice. Each host mouse was injected with 107 cells (4 × 106 B cells). Each point represents the number of donor LN-derived B cells (B220+ μ+) recovered in the SPL of three to six host mice as a function of time. The curve joins the mean of the values obtained at each time point. (B) Phenotype of in vivo activated B cells. Rag2−/− mice were injected with 4 × 107 LN cells (1.8 × 107 B cells). The hosts were killed 2 mo later and the SPL cells analyzed by flow cytometry. Histograms are gated for the B220+ B cell population. Bold line, phenotypic analysis of LN-derived B cells. Thin line, phenotypic analysis of splenic B cells from C57Bl/6 control mice. (C) Contour plot showing the sorted B2 cells from CD3ε−/− donors before and after in vivo activation in Rag2−/− hosts. Similar observations were made when LN cells from the same donors were transferred. (D) Cytokine profile of in vivo activated B cells. RT-PCR was performed on sorted B cells (>99% pure). Lanes from the left to the right: splenic cells from Rag2−/− mice injected 1 mo before with LN cells from CD3ε−/− donors (pool of 10 mice); mature B cells (IgMlowIgDhigh) from the SPL of CD3ε−/− mice (pool of 5 mice); activated B cells (IgMhighIgDlow) from the SPL of CD3ε−/− mice (pool of 5 mice); B cells from the LN of CD3ε−/− mice (pool of 5 mice). (E) Number of IgM- secreting cells in the SPL of immunodeficient hosts injected with 107 cells (4 × 106 B cells) as a function of time. Each point represents one individual mouse and the curve joins the mean values obtained in each time point.
Figure 1
(A) Fate of LN B cells transferred into immunodeficient Rag2−/− mice. Each host mouse was injected with 107 cells (4 × 106 B cells). Each point represents the number of donor LN-derived B cells (B220+ μ+) recovered in the SPL of three to six host mice as a function of time. The curve joins the mean of the values obtained at each time point. (B) Phenotype of in vivo activated B cells. Rag2−/− mice were injected with 4 × 107 LN cells (1.8 × 107 B cells). The hosts were killed 2 mo later and the SPL cells analyzed by flow cytometry. Histograms are gated for the B220+ B cell population. Bold line, phenotypic analysis of LN-derived B cells. Thin line, phenotypic analysis of splenic B cells from C57Bl/6 control mice. (C) Contour plot showing the sorted B2 cells from CD3ε−/− donors before and after in vivo activation in Rag2−/− hosts. Similar observations were made when LN cells from the same donors were transferred. (D) Cytokine profile of in vivo activated B cells. RT-PCR was performed on sorted B cells (>99% pure). Lanes from the left to the right: splenic cells from Rag2−/− mice injected 1 mo before with LN cells from CD3ε−/− donors (pool of 10 mice); mature B cells (IgMlowIgDhigh) from the SPL of CD3ε−/− mice (pool of 5 mice); activated B cells (IgMhighIgDlow) from the SPL of CD3ε−/− mice (pool of 5 mice); B cells from the LN of CD3ε−/− mice (pool of 5 mice). (E) Number of IgM- secreting cells in the SPL of immunodeficient hosts injected with 107 cells (4 × 106 B cells) as a function of time. Each point represents one individual mouse and the curve joins the mean values obtained in each time point.
Figure 1
(A) Fate of LN B cells transferred into immunodeficient Rag2−/− mice. Each host mouse was injected with 107 cells (4 × 106 B cells). Each point represents the number of donor LN-derived B cells (B220+ μ+) recovered in the SPL of three to six host mice as a function of time. The curve joins the mean of the values obtained at each time point. (B) Phenotype of in vivo activated B cells. Rag2−/− mice were injected with 4 × 107 LN cells (1.8 × 107 B cells). The hosts were killed 2 mo later and the SPL cells analyzed by flow cytometry. Histograms are gated for the B220+ B cell population. Bold line, phenotypic analysis of LN-derived B cells. Thin line, phenotypic analysis of splenic B cells from C57Bl/6 control mice. (C) Contour plot showing the sorted B2 cells from CD3ε−/− donors before and after in vivo activation in Rag2−/− hosts. Similar observations were made when LN cells from the same donors were transferred. (D) Cytokine profile of in vivo activated B cells. RT-PCR was performed on sorted B cells (>99% pure). Lanes from the left to the right: splenic cells from Rag2−/− mice injected 1 mo before with LN cells from CD3ε−/− donors (pool of 10 mice); mature B cells (IgMlowIgDhigh) from the SPL of CD3ε−/− mice (pool of 5 mice); activated B cells (IgMhighIgDlow) from the SPL of CD3ε−/− mice (pool of 5 mice); B cells from the LN of CD3ε−/− mice (pool of 5 mice). (E) Number of IgM- secreting cells in the SPL of immunodeficient hosts injected with 107 cells (4 × 106 B cells) as a function of time. Each point represents one individual mouse and the curve joins the mean values obtained in each time point.
Figure 1
(A) Fate of LN B cells transferred into immunodeficient Rag2−/− mice. Each host mouse was injected with 107 cells (4 × 106 B cells). Each point represents the number of donor LN-derived B cells (B220+ μ+) recovered in the SPL of three to six host mice as a function of time. The curve joins the mean of the values obtained at each time point. (B) Phenotype of in vivo activated B cells. Rag2−/− mice were injected with 4 × 107 LN cells (1.8 × 107 B cells). The hosts were killed 2 mo later and the SPL cells analyzed by flow cytometry. Histograms are gated for the B220+ B cell population. Bold line, phenotypic analysis of LN-derived B cells. Thin line, phenotypic analysis of splenic B cells from C57Bl/6 control mice. (C) Contour plot showing the sorted B2 cells from CD3ε−/− donors before and after in vivo activation in Rag2−/− hosts. Similar observations were made when LN cells from the same donors were transferred. (D) Cytokine profile of in vivo activated B cells. RT-PCR was performed on sorted B cells (>99% pure). Lanes from the left to the right: splenic cells from Rag2−/− mice injected 1 mo before with LN cells from CD3ε−/− donors (pool of 10 mice); mature B cells (IgMlowIgDhigh) from the SPL of CD3ε−/− mice (pool of 5 mice); activated B cells (IgMhighIgDlow) from the SPL of CD3ε−/− mice (pool of 5 mice); B cells from the LN of CD3ε−/− mice (pool of 5 mice). (E) Number of IgM- secreting cells in the SPL of immunodeficient hosts injected with 107 cells (4 × 106 B cells) as a function of time. Each point represents one individual mouse and the curve joins the mean values obtained in each time point.
Figure 2
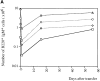
(A) Number of B cells recovered in the SPL of immunodeficient Rag2−/− mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); and ▵, 30 × 106 LN cells (9 × 106 B cells). (B) Number of IgM-secreting cells recovered in the SPL of immunodeficient mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B cells); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); ▵, 30 × 106 LN cells (9 × 106 B cells). (C) Number of B cells recovered in the SPL (○), the LN (▵), and the BM (⋄) as a function of the number of LN B cells injected into immunodeficient mice two mo after transfer (mean ± SD of three to six mice). (D) Phenotype of B cells recovered in the BM, SPL, and LN of the same immunodeficient mouse reconstituted with 4 × 107 LN B cells. In the SPL the majority of B cells show an activated phenotype (IgMhighIgDlow), whereas resting B cells (IgMlowIgDhigh) accumulate in the SPL, LN, and BM.
Figure 2
(A) Number of B cells recovered in the SPL of immunodeficient Rag2−/− mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); and ▵, 30 × 106 LN cells (9 × 106 B cells). (B) Number of IgM-secreting cells recovered in the SPL of immunodeficient mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B cells); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); ▵, 30 × 106 LN cells (9 × 106 B cells). (C) Number of B cells recovered in the SPL (○), the LN (▵), and the BM (⋄) as a function of the number of LN B cells injected into immunodeficient mice two mo after transfer (mean ± SD of three to six mice). (D) Phenotype of B cells recovered in the BM, SPL, and LN of the same immunodeficient mouse reconstituted with 4 × 107 LN B cells. In the SPL the majority of B cells show an activated phenotype (IgMhighIgDlow), whereas resting B cells (IgMlowIgDhigh) accumulate in the SPL, LN, and BM.
Figure 2
(A) Number of B cells recovered in the SPL of immunodeficient Rag2−/− mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); and ▵, 30 × 106 LN cells (9 × 106 B cells). (B) Number of IgM-secreting cells recovered in the SPL of immunodeficient mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B cells); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); ▵, 30 × 106 LN cells (9 × 106 B cells). (C) Number of B cells recovered in the SPL (○), the LN (▵), and the BM (⋄) as a function of the number of LN B cells injected into immunodeficient mice two mo after transfer (mean ± SD of three to six mice). (D) Phenotype of B cells recovered in the BM, SPL, and LN of the same immunodeficient mouse reconstituted with 4 × 107 LN B cells. In the SPL the majority of B cells show an activated phenotype (IgMhighIgDlow), whereas resting B cells (IgMlowIgDhigh) accumulate in the SPL, LN, and BM.
Figure 2
(A) Number of B cells recovered in the SPL of immunodeficient Rag2−/− mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); and ▵, 30 × 106 LN cells (9 × 106 B cells). (B) Number of IgM-secreting cells recovered in the SPL of immunodeficient mice as a function of time. Each curve corresponds to a group of mice (three to six individuals) injected with a particular number of cells: □, 106 LN cells (0.3 × 106 B cells); ⋄, 5 × 106 LN cells (1.5 × 106 B cells); ○, 10 × 106 LN cells (3 × 106 B cells); ▵, 30 × 106 LN cells (9 × 106 B cells). (C) Number of B cells recovered in the SPL (○), the LN (▵), and the BM (⋄) as a function of the number of LN B cells injected into immunodeficient mice two mo after transfer (mean ± SD of three to six mice). (D) Phenotype of B cells recovered in the BM, SPL, and LN of the same immunodeficient mouse reconstituted with 4 × 107 LN B cells. In the SPL the majority of B cells show an activated phenotype (IgMhighIgDlow), whereas resting B cells (IgMlowIgDhigh) accumulate in the SPL, LN, and BM.
Figure 3
(A) IgM/IgD staining on SPL B cells recovered in a Rag2−/− mouse injected with two LN populations sequentially. (Top) Relative proportion of Ly51 cells in IgMlowIgDhigh subpopulation (mature B cells). (Bottom) Relative proportion of Ly51 cells in IgMhighIgDlow subpopulation (activated B cells). Note that activated B cells (IgMhighIgDlow) are primarily Ly51−, thus derived from the first population injected. (B) IgMb allotype concentrations in mice injected with B6Ly51 LN population (Ly51, IgHb) either alone (○) or that also received B6IgHa LN population (Ly52, IgHa) 1 mo before (□). Mice were killed 1 mo after the transfer of the B6Ly51 LN population.
Figure 3
(A) IgM/IgD staining on SPL B cells recovered in a Rag2−/− mouse injected with two LN populations sequentially. (Top) Relative proportion of Ly51 cells in IgMlowIgDhigh subpopulation (mature B cells). (Bottom) Relative proportion of Ly51 cells in IgMhighIgDlow subpopulation (activated B cells). Note that activated B cells (IgMhighIgDlow) are primarily Ly51−, thus derived from the first population injected. (B) IgMb allotype concentrations in mice injected with B6Ly51 LN population (Ly51, IgHb) either alone (○) or that also received B6IgHa LN population (Ly52, IgHa) 1 mo before (□). Mice were killed 1 mo after the transfer of the B6Ly51 LN population.
Figure 4
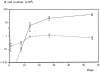
(A) Fate of LN-derived and BM-derived B cells in immunodeficient mice. Each host mouse was injected simultaneously with 4 × 106 LN B cells and 106 BM cells. Each point represents the number of donor LN-derived B cells (○) and BM-derived B cells (□) recovered in the SPL of three to six host mice as a function of time. The curves join the mean of the values obtained at each time point for each B cell population.
Similar articles
- Immunoglobulin M and D antigen receptors are both capable of mediating B lymphocyte activation, deletion, or anergy after interaction with specific antigen.
Brink R, Goodnow CC, Crosbie J, Adams E, Eris J, Mason DY, Hartley SB, Basten A. Brink R, et al. J Exp Med. 1992 Oct 1;176(4):991-1005. doi: 10.1084/jem.176.4.991. J Exp Med. 1992. PMID: 1402669 Free PMC article. - Reduced life span of anergic self-reactive B cells in a double-transgenic model.
Fulcher DA, Basten A. Fulcher DA, et al. J Exp Med. 1994 Jan 1;179(1):125-34. doi: 10.1084/jem.179.1.125. J Exp Med. 1994. PMID: 8270860 Free PMC article. - The rat B cell system: the anatomical localization of flow cytometry-defined B cell subpopulations.
Kroese FG, Butcher EC, Lalor PA, Stall AM, Herzenberg LA. Kroese FG, et al. Eur J Immunol. 1990 Jul;20(7):1527-34. doi: 10.1002/eji.1830200718. Eur J Immunol. 1990. PMID: 2143728 - Fidelity and infidelity in commitment to B-lymphocyte lineage development.
Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Rolink AG, et al. Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
Cited by
- Notch2 controls developmental fate choices between germinal center and marginal zone B cells upon immunization.
Babushku T, Lechner M, Ehrenberg S, Rambold U, Schmidt-Supprian M, Yates AJ, Rane S, Zimber-Strobl U, Strobl LJ. Babushku T, et al. Nat Commun. 2024 Mar 4;15(1):1960. doi: 10.1038/s41467-024-46024-1. Nat Commun. 2024. PMID: 38438375 Free PMC article. - B cell abnormalities and autoantibody production in patients with partial RAG deficiency.
Min Q, Csomos K, Li Y, Dong L, Hu Z, Meng X, Yu M, Walter JE, Wang JY. Min Q, et al. Front Immunol. 2023 Jul 5;14:1155380. doi: 10.3389/fimmu.2023.1155380. eCollection 2023. Front Immunol. 2023. PMID: 37475856 Free PMC article. Review. - Stromal Notch ligands foster lymphopenia-driven functional plasticity and homeostatic proliferation of naive B cells.
Gómez Atria D, Gaudette BT, Londregan J, Kelly S, Perkey E, Allman A, Srivastava B, Koch U, Radtke F, Ludewig B, Siebel CW, Ryan RJ, Robertson TF, Burkhardt JK, Pear WS, Allman D, Maillard I. Gómez Atria D, et al. J Clin Invest. 2022 Jul 1;132(13):e158885. doi: 10.1172/JCI158885. J Clin Invest. 2022. PMID: 35579963 Free PMC article. - Optimization of a human milk-directed quantitative sIgA ELISA method substantiated by mass spectrometry.
Dingess KA, van Dam P, Zhu J, Mank M, Knipping K, Heck AJR, Stahl B. Dingess KA, et al. Anal Bioanal Chem. 2021 Aug;413(20):5037-5049. doi: 10.1007/s00216-021-03468-4. Epub 2021 Jun 25. Anal Bioanal Chem. 2021. PMID: 34169348 Free PMC article. - Notch2-mediated plasticity between marginal zone and follicular B cells.
Lechner M, Engleitner T, Babushku T, Schmidt-Supprian M, Rad R, Strobl LJ, Zimber-Strobl U. Lechner M, et al. Nat Commun. 2021 Feb 17;12(1):1111. doi: 10.1038/s41467-021-21359-1. Nat Commun. 2021. PMID: 33597542 Free PMC article.
References
- Freitas AA, Rocha B, Coutinho A. Lymphocyte population kinetics in the mouse. Immunol Rev. 1986;91:5–37. - PubMed
- Opstelten D, Osmond DG. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu chain–bearing cells in normal mice. J Immunol. 1983;131:2635–2640. - PubMed
- Osmond DG. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986;93:103–124. - PubMed
- Forster I, Vieira P, Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1:321–331. - PubMed
- Rocha B, Penit C, Baron C, Vasseur F, Dautigny N, Freitas AA. Accumulation of bromodeoxyuridine- labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990;20:1697–1708. - PubMed