Plugging the leaks - PubMed (original) (raw)
Plugging the leaks
D A Goodenough. Proc Natl Acad Sci U S A. 1999.
No abstract available
Figures
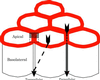
Figure 1
A diagram of six epithelial cells packed into a portion of an epithelium. The apical and basolateral membranes are separated by tight junctions (shown as continuous red rings joining the cells). Selective epithelial permeability involves both the transcellular and paracellular routes. In the former, solutes are transported across the apical plasma membrane, diffuse through the cytoplasm (dashed arrow), and are then retransported out of the cell into the extracellular space beneath the tight junctions. In the paracellular route, solutes move through channels in the tight junction itself (solid arrow) and gain access to the paracellular spaces without entering the cytoplasm of the cells. The small box indicates the plane of section shown in diagram in Fig. 3.
Figure 2
Electron microscopy of tight junctions in liver from mouse bile canaliculi. (A) Freeze-fracture image of the canaliculus angles across the field of view from lower left to upper right. The tight junctions appear as branching and anastomosing strands on the P fracture face (above the canaliculus) and complementary grooves on the E fracture face (below the canaliculus). (B) Thin-section image in which the tight junctions can be seen flanking the microvillus-filled canaliculus. Horseradish peroxidase was introduced into the extracellular spaces via the vascular system and is seen to penetrate the extracellular spaces but is denied access to the canalicular lumen by the tight junctions. (Bar = 1 μm.)
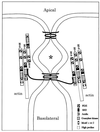
Figure 3
A diagram of the vertical section (small box) in Fig. 1. The apical and basolateral membranes are separated by one or more membrane–membrane interactions extending in the plane normal to the figure to form the paracellular seal. In three dimensions, these interactions would anastomose in this plane and then branch, creating the weblike strand network seen in freeze-fracture images in Fig. 2. Two interactions are drawn here: apical, showing a fanciful bonding between two claudin proteins via their extracellular loops, and basal, showing a similar bonding between two occludin proteins, whose longer cytoplasmic C termini interact with the guanylate kinase domain of either ZO-1 or ZO-3. Actin is known to interact with the proline-rich tail of ZO-1. Each ZO-1 is shown interacting with ZO-2 via their second PDZ domains. The claudins and occludins are drawn as monomers; presumably they are oligomerized with themselves or with each other in the membrane. ∗ is placed in a chamber created between two membrane–membrane interactions, which may be isolated from both the apical and basolateral compartments.
Comment on
- Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands.
Morita K, Furuse M, Fujimoto K, Tsukita S. Morita K, et al. Proc Natl Acad Sci U S A. 1999 Jan 19;96(2):511-6. doi: 10.1073/pnas.96.2.511. Proc Natl Acad Sci U S A. 1999. PMID: 9892664 Free PMC article.
Similar articles
- Dynamics and functions of tight junctions.
Steed E, Balda MS, Matter K. Steed E, et al. Trends Cell Biol. 2010 Mar;20(3):142-9. doi: 10.1016/j.tcb.2009.12.002. Epub 2010 Jan 12. Trends Cell Biol. 2010. PMID: 20061152 Review. - The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis.
Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. Raschperger E, et al. Exp Cell Res. 2006 May 15;312(9):1566-80. doi: 10.1016/j.yexcr.2006.01.025. Epub 2006 Mar 15. Exp Cell Res. 2006. PMID: 16542650 - Multiple domains of occludin are involved in the regulation of paracellular permeability.
Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Balda MS, et al. J Cell Biochem. 2000 Apr;78(1):85-96. J Cell Biochem. 2000. PMID: 10797568 - Tight junctions: molecular architecture and function.
Aijaz S, Balda MS, Matter K. Aijaz S, et al. Int Rev Cytol. 2006;248:261-98. doi: 10.1016/S0074-7696(06)48005-0. Int Rev Cytol. 2006. PMID: 16487793 Review. - Occludin and the functions of tight junctions.
Matter K, Balda MS. Matter K, et al. Int Rev Cytol. 1999;186:117-46. doi: 10.1016/s0074-7696(08)61052-9. Int Rev Cytol. 1999. PMID: 9770298 Review.
Cited by
- Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells.
Morita K, Sasaki H, Furuse M, Tsukita S. Morita K, et al. J Cell Biol. 1999 Oct 4;147(1):185-94. doi: 10.1083/jcb.147.1.185. J Cell Biol. 1999. PMID: 10508865 Free PMC article. - Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Ryeom SW, Paul D, Goodenough DA. Ryeom SW, et al. Mol Biol Cell. 2000 May;11(5):1687-96. doi: 10.1091/mbc.11.5.1687. Mol Biol Cell. 2000. PMID: 10793144 Free PMC article. - Downstream genes of Sox8 that would affect adult male fertility.
Singh AP, Harada S, Mishina Y. Singh AP, et al. Sex Dev. 2009;3(1):16-25. doi: 10.1159/000200078. Epub 2009 Apr 1. Sex Dev. 2009. PMID: 19339814 Free PMC article. - Permeability and route of entry for lipid-insoluble molecules across brain barriers in developing Monodelphis domestica.
Ek CJ, Habgood MD, Dziegielewska KM, Potter A, Saunders NR. Ek CJ, et al. J Physiol. 2001 Nov 1;536(Pt 3):841-53. doi: 10.1111/j.1469-7793.2001.00841.x. J Physiol. 2001. PMID: 11691876 Free PMC article. - Androgens regulate the permeability of the blood-testis barrier.
Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Meng J, et al. Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16696-700. doi: 10.1073/pnas.0506084102. Epub 2005 Nov 7. Proc Natl Acad Sci U S A. 2005. PMID: 16275920 Free PMC article.
References
- Boulpaep E L, Seely J F. Am J Physiol. 1971;221:1084–1096. - PubMed
- Frömter E, Diamond J M. Nature (London) 1972;235:9–13. - PubMed
- Pappenheimer J R. J Membr Biol. 1987;100:137–148. - PubMed
- Madara J L, Pappenheimer J R. J Membr Biol. 1987;100:149–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources