A role for RNA helicase A in post-transcriptional regulation of HIV type 1 - PubMed (original) (raw)
A role for RNA helicase A in post-transcriptional regulation of HIV type 1
J Li et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Retroviruses must bypass the tight coupling of splicing and nuclear export of mRNA in their replication cycle because unspliced genomic RNA and incompletely spliced mRNA must be exported to the cytoplasm for packaging or translation. This process is mediated by a cis-acting constitutive transport element (CTE) for simple retroviruses and by the trans-acting viral protein Rev in concert with its response element (RRE) for complex retroviruses (e.g., HIV). Recently, we identified RNA helicase A (RHA) as a potential cellular cofactor for CTE. Here, we report that RHA also plays a role in Rev/RRE-mediated gene expression and HIV replication. RHA binds weakly to HIV-1 RRE independently of Rev. Overexpression of RHA, but not of an RHA mutant lacking helicase activity, increased both Rev/RRE- and CTE-dependent gene expression and the levels of unspliced HIV mRNA. Microinjection of antibodies to RHA into nuclei dramatically inhibited both CTE- and Rev-dependent gene expression in human cells. Exogenous RHA cDNA, but not the mutant RHA, rescued this inhibition. We propose that RHA is required to release both CTE- and RRE-containing mRNA from spliceosomes before completion of splicing, thus freeing them for nuclear export.
Figures
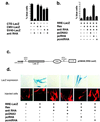
Figure 1
Interaction between RHA and RRE. (a) In vitro binding of RHA to CTE but not to RRE in the presence or absence of Rev. RNA Selection with biotin-labeled RRE (lanes 2, 3, and 6) or CTE (lane 4) was carried out as described (13). Recombinant Rev was added to the nuclear extract in some samples (lanes 2, 5, and 6). Lanes 1–4 were probed with anti-RHA antibodies and lanes 5–6 were probed with anti-Rev antibodies. Lane 1 (nuclear extract) and lane 5 (Rev mixed with nuclear extract) were positive controls for direct immunodetection with antibodies without RNA selection. The arrow indicates the position of the Rev-specific band in lanes 5 and 6. (b) Detection of in vivo interactions between RHA and RRE or Rev-RRE by using immunoprecipitation and RT-PCR. Lysates from cells transfected with pRev and pRRE (lanes 1–6) or pRRE alone (lanes 7–12) were immunoprecipitated with normal serum (lanes 1, 2, 7, and 8), anti-Rev antibodies (lane, 3, 4, 9, and 10), or anti-RHA antibodies (lanes 5, 6, 11, and 12). Half of the RNA obtained from the precipitate was subjected to RT (lanes 1, 3, 5, 7, 9, and 11) and PCR amplification using RRE-specific primers. The other half was processed under the same conditions without RT (lanes 2, 4, 6, 8, 10, and 12). The expected product for RRE was 230 bp in length.
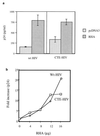
Figure 2
Microinjection of anti-RHA antibody blocks CTE or Rev/RRE-mediated reporter gene expression in human cells. (a) HS68 fibroblasts were injected with LacZ reporter constructs in which expression of β-galactosidase either was mediated by CTE or was driven constitutively by viral promoters. Constructs were coinjected with either preimmune IgG or anti-RHA as indicated. (b) Microinjection of a Rev-dependent RRE reporter construct with preimmune IgG or anti-RHA. Rescue experiments were conducted by coinjection of constructs encoding either wild-type RHA or an enzymatically inactive point mutant, mtRHA (14). The error bars represent standard errors. (c) Schematic representation of RRE reporter construct. (d) Phase contrast and corresponding immunofluorescence photomicrographs that demonstrated typical experimental results.
Figure 3
Increase of p24 production in virus producing cells cotransfected with RHA. (a) RHA increased the p24 production of HIV. HeLa cells were transfected with a plasmid containing wild-type HIV provirus or a CTE-containing modified HIV provirus (pNL43R-R-5). About 10× more (in milligrams) pcRHA or pcDNA3 was included in the transfection. p24 was measured on day 4 after transfection. The error bars indicate the range of values from two different experiments, and the average values were used in the graphs. (b) RHA dose-dependent increase of p24 production. HeLa cells were cotransfected with wild-type or CTE-modified HIV plus various amount of pcRHA. pcDNA3 was included to keep the total amount of DNA balanced at each point. p24 in culture medium was measured on day 4 after transfection.
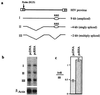
Figure 4
RHA enhanced the production of unspliced or singly spliced HIV RNA. (a) Structure of the three species of viral RNA. (b) Northern blot analysis of HIV RNA in cells cotransfected with HIV proviral DNA and pcRHA or pcDNA3. HeLa cells were transfected as described in Fig. 3_A_. Two days after transfection, total cellular RNA was extracted and subjected to Northern blot analysis using the RU5 sequence of HIV-1 LTR as probe. I, II, and III represent unspliced, singly spliced, and multiply spliced viral RNA, respectively. (c) Increased ratio of unspliced and singly spliced viral RNA to multiply spliced RNA in cells transfected with pcRHA. The amount of each viral RNA species was quantified by measuring the band intensity on the x-ray film by using image analysis software (
imagequant
, Modular Dynamics).
Similar articles
- Sam68, RNA helicase A and Tap cooperate in the post-transcriptional regulation of human immunodeficiency virus and type D retroviral mRNA.
Reddy TR, Tang H, Xu W, Wong-Staal F. Reddy TR, et al. Oncogene. 2000 Jul 27;19(32):3570-5. doi: 10.1038/sj.onc.1203676. Oncogene. 2000. PMID: 10951562 - The simian retrovirus-1 constitutive transport element, unlike the HIV-1 RRE, uses factors required for cellular mRNA export.
Saavedra C, Felber B, Izaurralde E. Saavedra C, et al. Curr Biol. 1997 Sep 1;7(9):619-28. doi: 10.1016/s0960-9822(06)00288-0. Curr Biol. 1997. PMID: 9285715 - Synergistic stimulation of HIV-1 rev-dependent export of unspliced mRNA to the cytoplasm by hnRNP A1.
Najera I, Krieg M, Karn J. Najera I, et al. J Mol Biol. 1999 Feb 5;285(5):1951-64. doi: 10.1006/jmbi.1998.2473. J Mol Biol. 1999. PMID: 9925777 - Nucleocytoplasmic RNA transport in retroviral replication.
Wodrich H, Kräusslich HG. Wodrich H, et al. Results Probl Cell Differ. 2001;34:197-217. doi: 10.1007/978-3-540-40025-7_12. Results Probl Cell Differ. 2001. PMID: 11288676 Review. - Retroviral RNA elements integrate components of post-transcriptional gene expression.
Boris-Lawrie K, Roberts TM, Hull S. Boris-Lawrie K, et al. Life Sci. 2001 Oct 26;69(23):2697-709. doi: 10.1016/s0024-3205(01)01360-1. Life Sci. 2001. PMID: 11720075 Review.
Cited by
- The Host DHX9 DExH-Box Helicase Is Recruited to Chikungunya Virus Replication Complexes for Optimal Genomic RNA Translation.
Matkovic R, Bernard E, Fontanel S, Eldin P, Chazal N, Hassan Hersi D, Merits A, Péloponèse JM Jr, Briant L. Matkovic R, et al. J Virol. 2019 Feb 5;93(4):e01764-18. doi: 10.1128/JVI.01764-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463980 Free PMC article. - The carboxyl terminus of RNA helicase A contains a bidirectional nuclear transport domain.
Tang H, McDonald D, Middlesworth T, Hope TJ, Wong-Staal F. Tang H, et al. Mol Cell Biol. 1999 May;19(5):3540-50. doi: 10.1128/MCB.19.5.3540. Mol Cell Biol. 1999. PMID: 10207077 Free PMC article. - Oncogenic fusion protein EWS-FLI1 is a network hub that regulates alternative splicing.
Selvanathan SP, Graham GT, Erkizan HV, Dirksen U, Natarajan TG, Dakic A, Yu S, Liu X, Paulsen MT, Ljungman ME, Wu CH, Lawlor ER, Üren A, Toretsky JA. Selvanathan SP, et al. Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1307-16. doi: 10.1073/pnas.1500536112. Epub 2015 Mar 3. Proc Natl Acad Sci U S A. 2015. PMID: 25737553 Free PMC article. - EBNA-LP associates with cellular proteins including DNA-PK and HA95.
Han I, Harada S, Weaver D, Xue Y, Lane W, Orstavik S, Skalhegg B, Kieff E. Han I, et al. J Virol. 2001 Mar;75(5):2475-81. doi: 10.1128/JVI.75.5.2475-2481.2001. J Virol. 2001. PMID: 11160753 Free PMC article. - RNA-protein hybrid ribozymes that efficiently cleave any mRNA independently of the structure of the target RNA.
Warashina M, Kuwabara T, Kato Y, Sano M, Taira K. Warashina M, et al. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5572-7. doi: 10.1073/pnas.091411398. Proc Natl Acad Sci U S A. 2001. PMID: 11344300 Free PMC article.
References
- Malim M H, Hauber J, Le S Y, Maizel J V, Cullen B R. Nature (London) 1989;338:254–257. - PubMed
- Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. Cell. 1995;82:475–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases