Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis - PubMed (original) (raw)
Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis
M W Tan et al. Proc Natl Acad Sci U S A. 1999.
Abstract
We show that a single clinical isolate of the human opportunistic pathogen Pseudomonas aeruginosa (strain PA14), which previously was shown to be pathogenic in mice and plants, also kills Caenorhabditis elegans. The rate of PA14-mediated killing of C. elegans depends on the composition of the agar medium on which PA14 is grown. When PA14 is grown on minimal medium, killing occurs over the course of several days and is referred to as "slow" killing. When PA14 is grown on high-osmolarity medium, killing occurs over the course of several hours and is referred to as "fast" killing. Several lines of evidence, including the fact that heat-killed bacteria are still capable of fast but not slow killing of C. elegans, indicate that fast and slow killing occur by distinct mechanisms. Slow killing involves an infection-like process and correlates with the accumulation of PA14 within worm intestines. Among 10 PA14 virulence-related mutants that had been shown previously to affect pathogenicity in plants and mice, 6 were less effective in killing C. elegans under both fast- and slow-killing conditions, indicating a high degree of commonalty among the P. aeruginosa factors required for pathogenicity in disparate eukaryotic hosts. Thus, we show that a C. elegans pathogenicity model that is genetically tractable from the perspectives of both host and pathogen can be used to model mammalian bacterial pathogenesis.
Figures
Figure 1
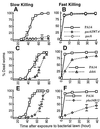
Kinetics of the killing of C. elegans by P. aeruginosa under slow-killing and fast-killing conditions. (A) L4 stage (open inverted triangles) and 1-day-old adult hermaphrodite (closed inverted triangles) worms feeding on P. aeruginosa PA14 grown on NG (slow killing) medium. (B) L4 stage (open inverted triangles) and 1-day-old adult hermaphrodite (closed inverted triangles) worms exposed to P. aeruginosa PA14 grown on PGS (fast killing) medium. (C) Adult (1-day-old) hermaphrodite C. elegans feeding on P. aeruginosa PA14 (open squares), PAO1 (open circles), PA29 (open diamonds), PAK (open triangles), and PO37 (crossed squares) grown on NG (slow killing) medium. (D) L4 stage C. elegans feeding on P. aeruginosa PA14 (open squares), PAO1 (open circles), PA29 (open diamonds), PAK (open triangles), and PO37 (crossed squares), and P. fluorescens 2-79 (crossed diamonds) grown on PGS (fast killing) medium. (E) L4 stage worms feeding on heat-killed PA14 (30 min at 65°C) grown on NG (open squares) or PGS (open circles) media or L4 stage worms feeding on live PA14 grown on NG (closed squares) or PGS (closed circles) media.
Figure 2
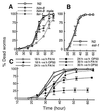
The kinetics of killing by P. aeruginosa mutants, gacA (A and B), _gacASW7_-4 (A and B), dsbA (C and D), and pho34B12 (E and F) compared with the parental wild-type PA14 (open squares) under slow-killing (Left) and fast-killing (Right) conditions.
Figure 3
Mechanisms of slow killing. (A) Death rates of nongravid adult worms. him-8 males (open circles) and fer-1 hermaphrodites (open diamonds) were compared with gravid adult worms, wild-type N2 hermaphrodites (open squares), and him-8 hermaphrodites (open triangles) feeding on PA14 grown on NG (slow killing) medium. (B) Mortality rates of 1-day-old adult wild-type N2 (open squares) and 1-day-old adult eat-1 (ad427) feeding on PA14 grown on NG medium. (C) C. elegans shifting experiments (see Materials and Methods) showing the average percentages of dead worms after transfer from PA14 to either OP50 or PA14: worms transferred to PA14 (open circles) or OP50 (closed circles) after feeding for 18 h on PA14; worms transferred to PA14 (open squares) or OP50 (closed squares) after feeding for 24 h on PA14; and worms transferred to PA14 (open triangles) or OP50 (closed triangles) after feeding for 30 h on PA14. Similar results were obtained when PA14 or PA14/GFP were shifted to DH5α or DH5α/GFP (not shown).
Figure 4
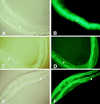
Fluorescence micrographs of worms fed on PA14/GFP (B), DH5α/GFP (D), and _gacA SW7_-4/GFP (F) for 48 h. The cellular structures of the same worms visualized under Nomarski phase contrast are shown in A, C, and D, respectively. (B) The entire lumen of worms fed on PA14/GFP was filled with GFP-expressing PA14. PA14/GFP began to accumulate after 36 h of feeding. In contrast, the lumen of worms feed on DH5α/GFP (D) and _gacA SW7_-4/GFP (F) showed no detectable GFP (see arrows), indicating that very few, if any, of the nonpathogenic bacteria were present. The intestinal cells were highly fluorescent in D and E because of autofluorescence. For _gacA SW7_-4/GFP, GFP expression was detected at 72 h. The micrographs for PA14/GFP are shown at ×400 magnification, whereas the micrographs for DH5α/GFP and _gacA SW7_-4/GFP are at ×160 magnification.
Similar articles
- Pseudomonas aeruginosa PA14 pathogenesis in Caenorhabditis elegans.
Kirienko NV, Cezairliyan BO, Ausubel FM, Powell JR. Kirienko NV, et al. Methods Mol Biol. 2014;1149:653-69. doi: 10.1007/978-1-4939-0473-0_50. Methods Mol Biol. 2014. PMID: 24818940 - The Pseudomonas aeruginosa PA14 type III secretion system is expressed but not essential to virulence in the Caenorhabditis elegans-P. aeruginosa pathogenicity model.
Wareham DW, Papakonstantinopoulou A, Curtis MA. Wareham DW, et al. FEMS Microbiol Lett. 2005 Jan 15;242(2):209-16. doi: 10.1016/j.femsle.2004.11.018. FEMS Microbiol Lett. 2005. PMID: 15621439 - Isolation and characterization of HepP: a virulence-related Pseudomonas aeruginosa heparinase.
Dzvova N, Colmer-Hamood JA, Griswold JA, Hamood AN. Dzvova N, et al. BMC Microbiol. 2017 Dec 16;17(1):233. doi: 10.1186/s12866-017-1141-0. BMC Microbiol. 2017. PMID: 29246112 Free PMC article. - The art of serendipity: killing of Caenorhabditis elegans by human pathogens as a model of bacterial and fungal pathogenesis.
Mylonakis E, Ausubel FM, Tang RJ, Calderwood SB. Mylonakis E, et al. Expert Rev Anti Infect Ther. 2003 Jun;1(1):167-73. doi: 10.1586/14787210.1.1.167. Expert Rev Anti Infect Ther. 2003. PMID: 15482109 Review. - Caenorhabditis elegans: a model genetic host to study Pseudomonas aeruginosa pathogenesis.
Tan MW, Ausubel FM. Tan MW, et al. Curr Opin Microbiol. 2000 Feb;3(1):29-34. doi: 10.1016/s1369-5274(99)00047-8. Curr Opin Microbiol. 2000. PMID: 10679415 Review.
Cited by
- Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa.
Kirienko NV, Ausubel FM, Ruvkun G. Kirienko NV, et al. Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):1821-6. doi: 10.1073/pnas.1424954112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624506 Free PMC article. - Caenorhabditis elegans, a model organism for investigating immunity.
Marsh EK, May RC. Marsh EK, et al. Appl Environ Microbiol. 2012 Apr;78(7):2075-81. doi: 10.1128/AEM.07486-11. Epub 2012 Jan 27. Appl Environ Microbiol. 2012. PMID: 22286994 Free PMC article. Review. - Roles of RcsA, an AhpD Family Protein, in Reactive Chlorine Stress Resistance and Virulence in Pseudomonas aeruginosa.
Nontaleerak B, Duang-Nkern J, Wongsaroj L, Trinachartvanit W, Romsang A, Mongkolsuk S. Nontaleerak B, et al. Appl Environ Microbiol. 2020 Oct 1;86(20):e01480-20. doi: 10.1128/AEM.01480-20. Print 2020 Oct 1. Appl Environ Microbiol. 2020. PMID: 32801171 Free PMC article. - Antibiotics Trigger Host Innate Immune Response via Microbiota-Brain Communication in C. elegans.
Wu Y, Li G, Tang H. Wu Y, et al. Int J Mol Sci. 2024 Aug 14;25(16):8866. doi: 10.3390/ijms25168866. Int J Mol Sci. 2024. PMID: 39201552 Free PMC article. - Recognition of familiar food activates feeding via an endocrine serotonin signal in Caenorhabditis elegans.
Song BM, Faumont S, Lockery S, Avery L. Song BM, et al. Elife. 2013 Feb 5;2:e00329. doi: 10.7554/eLife.00329. Elife. 2013. PMID: 23390589 Free PMC article.
References
- Ghosh S, Baltimore D. Nature (London) 1990;344:678–682. - PubMed
- Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr Nature (London) 1997;388:394–397. - PubMed
- Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann J A. Cell. 1996;86:973–983. - PubMed
- Hammond-Kosack K E, Jones J D G. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:575–607. - PubMed
- Ganz T, Lehrer R I. Curr Opin Immunol. 1994;6:584–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources