Failure of spinal cord oligodendrocyte development in mice lacking neuregulin - PubMed (original) (raw)
Failure of spinal cord oligodendrocyte development in mice lacking neuregulin
T Vartanian et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Oligodendrocytes develop from a subpopulation of precursor cells within the ventral ventricular zone of the spinal cord. The molecular cues that direct this spatially and temporally restricted event seem to originate in part from structures ventral to and within the spinal cord. Here, we present evidence that the family of ligands termed neuregulins are necessary for the normal generation of mouse spinal cord oligodendrocytes. Oligodendrocytes mature in spinal cord explants from wild-type mice and mice heterozygotic for a null mutation in the neuregulin gene (NRG +/-) in a temporal sequence of developmental events that replicates that observed in vivo. However, in spinal cord explants derived from mice lacking neuregulin (NRG -/-), oligodendrocytes fail to develop. Addition of recombinant neuregulin to spinal cord explants from NRG -/- mice rescues oligodendrocyte development. In wild-type spinal cord explants, inhibitors of neuregulin mimic the inhibition of oligodendrocyte development that occurs in NRG -/- explants. In embryonic mouse spinal cord, neuregulins are present in motor neurons and the ventral ventricular zone where they likely exert their influence on early oligodendrocyte precursor cells.
Figures
Figure 1
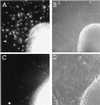
Oligodendrocytes fail to develop in spinal cord explants from neuregulin knock-out mice. Timed pregnancies from NRG +/− female and NRG +/− male matings were carried to ≈9.5 dpc, at which time females were killed, and embryos were removed. Each embryo was assigned a code number used to blind investigators from the genotyping. Genotyping was carried out by PCR on DNA extracted from the head of each embryo. Spinal cords were excised from embryos, cut transversely into segments, plated onto poly-
l
-lysine- and laminin-coated cover glasses, and cultured for 9 days in DMEM supplemented with 1% fetal bovine serum, N2 additives, and 10 ng/ml platelet-derived growth factor-AA. Cultures were stained with mAb O4 as described (32). Genotyping was unblinded after assessment of oligodendrocyte numbers and photography of explant cultures. (A and B) Micrographs of explants from NRG +/− embryos. (C and D) Micrographs of explants from NRG −/− embryos. O4 staining images are shown on the Left adjacent to the corresponding phase-contrast images on the Right. There are no identifiable O4+ cells in explants from NRG −/− embryos (the fluorescence within the explant is background not cellular staining). In contrast, explants from NRG +/− embryos have numerous O4+ oligodendrocytes. NRG −/− explants do, however, have an extensive neuritic outgrowth and growth of cells other than oligodendrocytes as seen in the phase-contrast images.
Figure 2
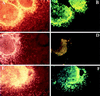
Exogenous recombinant neuregulin will rescue oligodendrocyte development from NRG −/− explant cultures. Spinal cord explant cultures were generated from litters ≈9.5 dpc and genotyped as described in the legend to Fig. 1. Spinal cords initially were divided into six to eight segments. Segments that did not obviously contain both ventral and dorsal spinal cord were discarded. Explants from a single embryo received either 0.1% BSA in PBS as a control or 1 nM neuregulin (epidermal-growth-factor-like β1 domain) at the time of plating. Explant cultures were then allowed to grow for an additional 8 days, equivalent to ≈17 dpc, and processed for O4 staining as described in the legend to Fig. 1. (C–F) Micrographs of explant cultures from NRG −/− embryos. (A and B) Micrographs of explant cultures from a wild-type littermate. The NRG −/− explants (C and D) as well as the wild-type explants (A and B) received 0.1% BSA in PBS. The explants shown in E and F received 1 nM neuregulin for the entire in vitro period. O4 staining images are shown on the Right adjacent to the corresponding phase-contrast images on the Left. The addition of neuregulin to NRG −/− explants restores oligodendrocyte development, and cells are indistinguishable from those in wild-type explants.
Figure 3
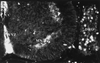
Neutralizing neuregulin activity inhibits the formation of oligodendrocytes in wild-type spinal cord explant cultures. IgB4 is a chimeric protein consisting of the extracellular domain of erbB4 (ligand-binding domain) and the Fc portion of human IgG1. Spinal cord explants were generated from E9.5 wild-type mice and cultured for 2.5 days in standard growth medium then 9 days in the presence of human Fc fragment as a control (a–d) or IgB4 (e–h). At day 11, cultures were surface stained with mAb O4 and O1 combined to identify immature and mature oligodendrocytes, fixed, and, in some cases, double-labeled with antineurofilament. (a, c, e, g) Combined O4 and O1 staining. b, d, and f are Nomarski images corresponding to a, c, and e, respectively. h shows the neurofilament staining corresponding to g. In cultures treated with IgB4, there are few or no oligodendrocytes identified. In contrast, wild-type explant cultures treated with the control buffer containing human Fc fragment had abundant numbers of oligodendrocytes.
Figure 4
Neuregulin is present in developing mouse spinal cord within motor neurons and the VVZ. E14 mouse embryos were emersion fixed in 4% paraformaldehyde, cryoprotected, and mounted in TissueTec, and 20-μm frozen transverse sections were mounted on glass slides. Sections were stained with a rabbit polyclonal antiserum that recognizes the “a” cytoplasmic domain of neuregulin, detected by fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antiserum, and visualized by epifluorescence. Neuregulin is present within motor neurons (MN), dorsal root ganglion neurons (DRG), and in the VVZ itself. CC = central canal.
Similar articles
- Local control of oligodendrocyte development in isolated dorsal mouse spinal cord.
Sussman CR, Dyer KL, Marchionni M, Miller RH. Sussman CR, et al. J Neurosci Res. 2000 Feb 1;59(3):413-20. doi: 10.1002/(SICI)1097-4547(20000201)59:3<413::AID-JNR16>3.0.CO;2-G. J Neurosci Res. 2000. PMID: 10679778 - The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes.
Park SK, Miller R, Krane I, Vartanian T. Park SK, et al. J Cell Biol. 2001 Sep 17;154(6):1245-58. doi: 10.1083/jcb.200104025. J Cell Biol. 2001. PMID: 11564761 Free PMC article. - Dorsal spinal cord inhibits oligodendrocyte development.
Wada T, Kagawa T, Ivanova A, Zalc B, Shirasaki R, Murakami F, Iemura S, Ueno N, Ikenaka K. Wada T, et al. Dev Biol. 2000 Nov 1;227(1):42-55. doi: 10.1006/dbio.2000.9869. Dev Biol. 2000. PMID: 11076675 - Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons.
Richardson WD, Pringle NP, Yu WP, Hall AC. Richardson WD, et al. Dev Neurosci. 1997;19(1):58-68. doi: 10.1159/000111186. Dev Neurosci. 1997. PMID: 9078434 Review. - Regulation of oligodendrocyte development.
Kagawa T, Wada T, Ikenaka K. Kagawa T, et al. Microsc Res Tech. 2001 Mar 15;52(6):740-5. doi: 10.1002/jemt.1058. Microsc Res Tech. 2001. PMID: 11276126 Review.
Cited by
- Intrinsic and extrinsic regulators of oligodendrocyte progenitor proliferation and differentiation.
Adams KL, Dahl KD, Gallo V, Macklin WB. Adams KL, et al. Semin Cell Dev Biol. 2021 Aug;116:16-24. doi: 10.1016/j.semcdb.2020.10.002. Epub 2020 Oct 22. Semin Cell Dev Biol. 2021. PMID: 34110985 Free PMC article. - Axonal control of oligodendrocyte development.
Barres BA, Raff MC. Barres BA, et al. J Cell Biol. 1999 Dec 13;147(6):1123-8. doi: 10.1083/jcb.147.6.1123. J Cell Biol. 1999. PMID: 10601327 Free PMC article. Review. No abstract available. - Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system.
Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Müller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Brinkmann BG, et al. Neuron. 2008 Aug 28;59(4):581-95. doi: 10.1016/j.neuron.2008.06.028. Neuron. 2008. PMID: 18760695 Free PMC article. - Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex.
Schmid RS, McGrath B, Berechid BE, Boyles B, Marchionni M, Sestan N, Anton ES. Schmid RS, et al. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4251-6. doi: 10.1073/pnas.0630496100. Epub 2003 Mar 20. Proc Natl Acad Sci U S A. 2003. PMID: 12649319 Free PMC article. - Akt-mediated survival of oligodendrocytes induced by neuregulins.
Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Flores AI, et al. J Neurosci. 2000 Oct 15;20(20):7622-30. doi: 10.1523/JNEUROSCI.20-20-07622.2000. J Neurosci. 2000. PMID: 11027222 Free PMC article.
References
- Meyer D, Birchmeier C. Nature (London) 1995;378:386–390. - PubMed
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Nature (London) 1995;378:390–394. - PubMed
- Lee K F, Simon H, Chen H, Bates B, Hung M C, Hauser C. Nature (London) 1995;378:394–398. - PubMed
- Sandrock A W J, Dryer S E, Rosen K M, Gozani S N, Kramer R, Theill L E, Fischbach G D. Science. 1997;276:599–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases