Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides - PubMed (original) (raw)
Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides
J P Greenfield et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The excessive generation and accumulation of 40- and 42-aa beta-amyloid peptides (Abeta40/Abeta42) in selectively vulnerable brain regions is a major neuropathological feature of Alzheimer's disease. Abeta, derived by proteolytic cleavage from the beta-amyloid precursor protein (betaAPP), is normally secreted. However, recent evidence suggests that significant levels of Abeta also may remain inside cells. Here, we have investigated the subcellular compartments within which distinct amyloid species are generated and the compartments from which they are secreted. Three experimental approaches were used: (i) immunofluorescence performed in intact cortical neurons; (ii) sucrose gradient fractionation performed with mouse neuroblastoma cells stably expressing wild-type betaAPP695 (N2a695); and (iii) cell-free reconstitution of Abeta generation and trafficking from N2a695 cells. These studies demonstrate that: (i) Abeta40 (Abeta1-40 plus Abetax-40, where x is an NH2-terminal truncation) is generated exclusively within the trans-Golgi Network (TGN) and packaged into post-TGN secretory vesicles; (ii) Abetax-42 is made and retained within the endoplasmic reticulum in an insoluble state; (iii) Abeta42 (Abeta1-42 plus Abetax-42) is made in the TGN and packaged into secretory vesicles; and (iv) the amyloid peptides formed in the TGN consist of two pools (a soluble population extractable with detergents and a detergent-insoluble form). The identification of the organelles in which distinct forms of Abeta are generated and from which they are secreted should facilitate the identification of the proteolytic enzymes responsible for their formation.
Figures
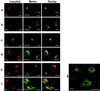
Figure 1
Localization of Aβ in rat primary neurons by double immunofluorescence confocal microscopy. (a and b) Aβ40 colocalizes with TGN38, but not with calnexin. (c and d) Aβ42 colocalizes with TGN-38 and calnexin. (e and f) Aβ1-x colocalizes with TGN38, but not calnexin. (g) EEA1 has a punctate, cytoplasmic localization, different than that of Aβ40, Aβ42, and Aβ1-x. Aβ were visualized by incubation with primary antibody followed by rhodamine-conjugated (red fluorescence) secondary antibody. TGN-38, calnexin, and EEA1 were used as markers for TGN, ER, and endsomes respectively, and were visualized by incubation with primary antibody followed by fluorescein isothiocyanate-conjugated (green fluorescence) secondary antibody. Overlays represent digitally merged images. Yellow fluorescence indicates colocalization of β-amyloid with marker protein. (Bar = 10 μm.)
Figure 2
Localization of Aβ by sucrose gradient fractionation. Rat primary neurons (a) and N2a cells doubly expressing human βAPP695 and wild-type PS1 (b and c) were homogenized, and a postnuclear supernatant was fractionated on an equilibrium flotation sucrose gradient (see Materials and Methods). Proteins from each fraction were precipitated with trichloroacetic acid and separated by 4–12% SDS/PAGE, followed by immunoblotting using antibodies against either (a) TGN-38, (b) BIP, or (c) βAPP. (d and e) N2a cells were labeled with [35S]methionine for 4 h at 37°C followed by fractionation as for _a_-c. Each fraction was sequentially immunoprecipitated with (d) FCA3340 (anti-Aβ40) and (e) FCA3642 (anti-Aβ42) and analyzed by 10–20% Tricine SDS/PAGE. Arrows indicate mature (m) and immature (im) forms of βAPP, Aβ1–40, Aβx-40, Aβ1–42, and Aβx-42.
Figure 3
Cell-free formation of post-ER and post-TGN vesicles containing βAPP. N2a cells were labeled with [35S]methionine either at 15°C for 4 h or at 37°C for 15 min followed by a 2-h chase at 20°C to accumulate labeled βAPP in the ER and TGN, respectively. Cells were permeabilized and incubated (see Materials and Methods) for 90 min with an energy-regenerating system at 15°C (lanes 1 and 3), 20°C (lanes 5 and 7), or 37°C (lanes 2, 4, 6, and 8). After incubation, samples were centrifuged, pellets (lanes 1, 2, 5, and 6) and supernatants (lanes 3, 4, 7, and 8) were immunoprecipitated with polyclonal antibody 369 and separated by SDS/PAGE (4–12%). Arrows indicate the positions of mature and immature βAPP.
Figure 4
Formation of Aβ40 and Aβ42 in a cell-free system. Experiments were performed as described in the legend to Fig. 3. except that, after detergent extraction, insoluble pellets were re-extracted by using 70% formic acid. Detergent-soluble (lanes 1 and 2), detergent-insoluble (lanes 3 and 4), and vesicle-associated (lanes 5 and 6) fractions were analyzed for Aβ40 (a and b) and Aβ42 (c and d) by sequential immunoprecipitation with FCA3440 followed by FCA3642. Immunoprecipitated proteins were subjected to Tricine SDS/PAGE (10–20%) analysis. Arrows indicate the positions of 4-kDa Aβ1–40, 3-kDa Aβx-40, 4-kDa Aβ1–42, and 3-kDa Aβx-42.
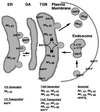
Figure 5
Proposed sites of Aβ generation in the secretory pathway. When βAPP is translocated into the lumen of the ER, an N-terminally truncated Aβ42 is formed by the actions of both a β-like secretase (s) and γ42-secretase. This Aβ42 remains in the ER in an insoluble state whereas uncleaved βAPP molecules are packaged into post-ER vesicles and travel through the Golgi apparatus (GA) to the TGN where most of them reside. Both β- and β-like secretases together with γ40- and γ42-secretases cleave βAPP within the TGN. Detergent-insoluble Aβ molecules aggregate and remain within the TGN. Full-length βAPP, β-CTFs, and soluble Aβ are packaged into post-TGN secretory vesicles. Full-length βAPP can be proteolyzed by α-secretase late within the secretory pathway/cell surface to release sβAPPα. Some uncleaved βAPP molecules as well as C-terminal fragments can be internalized via a clathrin-coated endocytic pathway to the endosome or lysosome where βAPP processing enzymes also might occur. A recently characterized pathway (23) could deliver those molecules to the TGN for an additional round of Aβ generation.
Similar articles
- Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.
Xia W, Zhang J, Ostaszewski BL, Kimberly WT, Seubert P, Koo EH, Shen J, Selkoe DJ. Xia W, et al. Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412 - Subcellular compartment and molecular subdomain of beta-amyloid precursor protein relevant to the Abeta 42-promoting effects of Alzheimer mutant presenilin 2.
Iwata H, Tomita T, Maruyama K, Iwatsubo T. Iwata H, et al. J Biol Chem. 2001 Jun 15;276(24):21678-85. doi: 10.1074/jbc.M007989200. Epub 2001 Mar 30. J Biol Chem. 2001. PMID: 11283011 - Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling.
Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Gasparini L, et al. J Neurosci. 2001 Apr 15;21(8):2561-70. doi: 10.1523/JNEUROSCI.21-08-02561.2001. J Neurosci. 2001. PMID: 11306609 Free PMC article. - Distinct presenilin-dependent and presenilin-independent gamma-secretases are responsible for total cellular Abeta production.
Wilson CA, Doms RW, Lee VM. Wilson CA, et al. J Neurosci Res. 2003 Nov 1;74(3):361-9. doi: 10.1002/jnr.10776. J Neurosci Res. 2003. PMID: 14598312 Review. - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
Cited by
- The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics.
Kent SA, Spires-Jones TL, Durrant CS. Kent SA, et al. Acta Neuropathol. 2020 Oct;140(4):417-447. doi: 10.1007/s00401-020-02196-w. Epub 2020 Jul 29. Acta Neuropathol. 2020. PMID: 32728795 Free PMC article. Review. - Key factors in Alzheimer's disease: beta-amyloid precursor protein processing, metabolism and intraneuronal transport.
Bayer TA, Wirths O, Majtényi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Bayer TA, et al. Brain Pathol. 2001 Jan;11(1):1-11. doi: 10.1111/j.1750-3639.2001.tb00376.x. Brain Pathol. 2001. PMID: 11145195 Free PMC article. Review. - Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins.
VanSlyke JK, Deschenes SM, Musil LS. VanSlyke JK, et al. Mol Biol Cell. 2000 Jun;11(6):1933-46. doi: 10.1091/mbc.11.6.1933. Mol Biol Cell. 2000. PMID: 10848620 Free PMC article. - Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway.
Das U, Scott DA, Ganguly A, Koo EH, Tang Y, Roy S. Das U, et al. Neuron. 2013 Aug 7;79(3):447-60. doi: 10.1016/j.neuron.2013.05.035. Neuron. 2013. PMID: 23931995 Free PMC article. - Aβ internalization by neurons and glia.
Mohamed A, Posse de Chaves E. Mohamed A, et al. Int J Alzheimers Dis. 2011 Feb 15;2011:127984. doi: 10.4061/2011/127984. Int J Alzheimers Dis. 2011. PMID: 21350608 Free PMC article.
References
- Goate A, Chartier-Harlin M, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Nature (London) 1991;349:704–706. - PubMed
- Murrell J, Farlow M, Ghetti B, Benson M D. Science. 1991;254:97–99. - PubMed
- Sherrington R, Rogaev E, Liang Y, Rogaeva E, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. - PubMed
- Levy-Lahad E, Wasco W, Poorkaj P, Romano D, Oshima J, Pettingel W H, Yu C-E, Jondro P D, Schmidt S D, Wang K, et al. Science. 1995;269:973–977. - PubMed
- Duff K, Eckman C, Zehr C, Yu X, Prada C, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Nature (London) 1996;383:710–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous