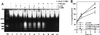Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity - PubMed (original) (raw)
Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity
L Y Gao et al. Infect Immun. 1999 Feb.
Abstract
The hallmark of Legionnaires' disease is intracellular replication of Legionella pneumophila within cells in the alveolar spaces. Cytopathogenicity of this bacterium to the host cell has been well demonstrated, but the mechanisms of host cell death due to infection by L. pneumophila are not well understood. In this study, induction of apoptosis in macrophages and alveolar epithelial cells by L. pneumophila during early stages of infection was confirmed by using multiple criteria, including DNA fragmentation by agarose gel electrophoresis, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, surface exposure of phosphatidylserine, and cellular morphology by transmission electron microscopy. Induction of nuclear apoptosis in L. pneumophila-infected macrophages is mediated by activation of the caspase cascade death machinery. We provide genetic and biochemical evidence that L. pneumophila-induced apoptosis in macrophages and alveolar epithelial cells does not require intracellular bacterial replication or new protein synthesis. In addition, extracellular L. pneumophila is capable of inducing apoptosis. Furthermore, induction of apoptosis by L. pneumophila correlates with cytopathogenicity. We conclude that L. pneumophila-induced apoptosis in macrophages and alveolar epithelial cells plays an important role in cytopathogenicity to the host cell during early stages of infection.
Figures
FIG. 1
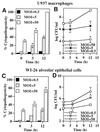
L. pneumophila is cytopathogenic to U937 macrophages (A) and WI-26 alveolar epithelial cells (C) in a dose-dependent manner. The cells were infected by the bacteria at an MOI of 0.5, 5, or 50 for 1 h, and the extracellular bacteria were washed away. The time points indicate the times at which Alamar Blue was added. The values are means of triplicate samples, and the error bars represent standard deviations. Intracellular growth kinetics of strain AA100 within U937 macrophages and WI-26 alveolar epithelial cells are shown in panels B and D, respectively. Infection of the monolayers was performed exactly as for cytopathogenicity assays, except that at the end of the 1-h infection period the monolayers were treated with gentamicin for 1 h to kill extracellular bacteria. At several time intervals, infected cells were hypotonically lysed and the number of intracellular bacteria was determined following growth on agar plates.
FIG. 2
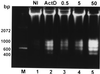
Kinetics of DNA fragmentation in _L. pneumophila_-infected (lanes 3 to 5) or ActD-treated (lane 2) U937 macrophages examined by agarose gel electrophoresis. The monolayers were infected by strain AA100 at an MOI of 0.5, 5, or 50 exactly as described in the legend to Fig. 1A. DNA isolated from uninfected or infected cells at 3 h post-1-h infection or 4 h after ActD treatment was subjected to electrophoresis and stained with ethidium bromide. Lane M contained a 100-bp molecular size marker (Gibco BRL, Gaithersburg, Md.). Lane 2 contained DNA from cells incubated with 1-μg/ml ActD. NI, noninfected.
FIG. 3
Apoptosis in _L. pneumophila_-infected U937 macrophages (A) and WI-26 alveolar epithelial cells (B) quantitated by TUNEL (see Fig. 4). The monolayers were infected by L. pneumophila as described in the legend to Fig. 2. One hundred cells were counted randomly, and the percentages of TUNEL-positive cells were calculated and expressed as mean percentages of apoptotic cells. NI, noninfected.
FIG. 4
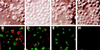
Representative confocal laser scanning images of TUNEL of _L. pneumophila_-infected U937 macrophages undergoing apoptosis. L. pneumophila infection and ActD treatment of the monolayers were performed exactly as described in the legend to Fig. 2. Monolayers were labeled simultaneously (bottom panels) with FITC-conjugated dUTP (green) and a polyclonal antiserum specific for L. pneumophila detected by a secondary antibody conjugated to Alexa red (red). Panels: B, L. pneumophila infected; D, L. pneumophila infected in the presence of CytD; F, ActD treated; H, uninfected. Phase-contrast images A, C, E, and G correspond to B, D, F, and H, respectively.
FIG. 5
Detection of _L. pneumophila_-induced apoptosis in U937 macrophages by transmission electron microscopy. Infected cells at 4 h postinfection (B) showed apoptotic nuclear morphology distinct from that of uninfected cells (A). The arrowheads indicate ribosome-surrounded phagosomes containing the bacteria. The arrows indicate intact mitochondria within the vicinity of the phagosomes.
FIG. 6
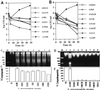
Representative confocal laser scanning images of annexin-V labeling of _L. pneumophila_-infected U937 macrophages undergoing apoptosis and comparison to ActD-treated cells. The Monolayers were labeled simultaneously with PI and FITC-conjugated annexin-V. Panels: A, 2 h postinfection; B, 3 h postinfection; C, 4 h post-ActD treatment; D, uninfected cells. The arrowheads indicate double-labeled cells, the arrows in panels B and C indicate cells labeled with PI only, and the rest of the fluorescent cells were with annexin-V only. Quantitation of annexin-V-positive and PI-positive U937 macrophages is shown in panel E. One hundred cells were counted randomly, and multiple samples were examined.
FIG. 7
Ability of pmi mutants of L. pneumophila to induce apoptosis in U937 macrophages correlates with cytopathogenicity to the cells. Intracellular growth kinetics of the mutants (A and B) was examined as described in the legend to Fig. 1B but without gentamicin treatment. The ability of these mutants to induce DNA fragmentation in U937 macrophages was examined at 3 h postinfection (C and D). Infection of the monolayers was performed at an MOI of 50 exactly as described in the legend to Fig. 1A. Percent cytopathogenicity of the wild-type and mutant strains of L. pneumophila to U937 macrophages infected at an MOI of 50 at 12 h postinfection is shown in panels E and F and was determined as described in the legend to Fig. 1A. Lane M contained a 100-bp molecular size marker. NI, noninfected.
FIG. 8
Inhibition of intracellular bacterial replication and inhibition of bacterial uptake do not block _L. pneumophila_-induced apoptosis and cytopathogenicity to U937 macrophages. (A) Some monolayers were treated with chloramphenicol at 20 (lane 7) or 100 (lane 8) μg/ml for 1 h prior to infection at an MOI of 50 and during the 1-h infection period and the subsequent 3-h incubation time. Some monolayers were pretreated with 1-μg/ml CytD for 30 min and infected at an MOI of 50 for 30 min in the presence of CytD before gentamicin treatment. CytD was either removed (lane 5) or maintained (lane 6) after the 1-h gentamicin treatment. Uninfected monolayers were incubated with chloramphenicol at 0 (lane 1), 20 (lane 9), or 100 (lane 10) μg/ml for 5 h or with CytD at 1 μg/ml for 1 h (lane 2) or 5 h (lane 3). Formalin-killed bacteria were also used to infect U937 macrophages to examine their ability to induce DNA fragmentation (lane 11). Lane M contained a 100-bp molecular size marker. (B) Cytopathogenicity of L. pneumophila to U937 macrophages under different treatment conditions. Infection or treatments were performed as described in the legend to Fig. 7 for detection of DNA fragmentation. Percent cytopathogenicity was determined as described in the legend to Fig. 1A.
FIG. 9
Inhibition of DNA fragmentation in _L. pneumophila_-infected U937 macrophages by the caspase inhibitor Z-VAD-FMK. Monolayers were pretreated with a 100 μM concentration of the inhibitor for 90 min and infected with strain AA100 at an MOI of 50 in the presence of the inhibitor, extracellular bacteria were washed off, and monolayers were incubated for an additional 3 h in the presence of the inhibitor before DNA isolation. Lane M contained a 100-bp molecular size marker. NI, noninfected.
Similar articles
- Activation of caspase 3 during Legionella pneumophila-induced apoptosis.
Gao LY, Abu Kwaik Y. Gao LY, et al. Infect Immun. 1999 Sep;67(9):4886-94. doi: 10.1128/IAI.67.9.4886-4894.1999. Infect Immun. 1999. PMID: 10456945 Free PMC article. - Heterogeneity in intracellular replication and cytopathogenicity of Legionella pneumophila and Legionella micdadei in mammalian and protozoan cells.
Gao LY, Susa M, Ticac B, Abu Kwaik Y. Gao LY, et al. Microb Pathog. 1999 Nov;27(5):273-87. doi: 10.1006/mpat.1999.0308. Microb Pathog. 1999. PMID: 10545255 - Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila.
Abu-Zant A, Santic M, Molmeret M, Jones S, Helbig J, Abu Kwaik Y. Abu-Zant A, et al. Infect Immun. 2005 Sep;73(9):5339-49. doi: 10.1128/IAI.73.9.5339-5349.2005. Infect Immun. 2005. PMID: 16113249 Free PMC article. - Molecular and cell biology of Legionella pneumophila.
Bitar DM, Molmeret M, Abu Kwaik Y. Bitar DM, et al. Int J Med Microbiol. 2004 Apr;293(7-8):519-27. doi: 10.1078/1438-4221-00286. Int J Med Microbiol. 2004. PMID: 15149027 Review. - [Immunopathogenesis of Legionnaires' disease].
Alim A. Alim A. Mikrobiyol Bul. 2004 Jul;38(3):295-303. Mikrobiyol Bul. 2004. PMID: 15490851 Review. Turkish.
Cited by
- Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication.
Lai XH, Golovliov I, Sjöstedt A. Lai XH, et al. Infect Immun. 2001 Jul;69(7):4691-4. doi: 10.1128/IAI.69.7.4691-4694.2001. Infect Immun. 2001. PMID: 11402018 Free PMC article. - Striking a balance: modulation of host cell death pathways by legionella pneumophila.
Luo ZQ. Luo ZQ. Front Microbiol. 2011 Feb 23;2:36. doi: 10.3389/fmicb.2011.00036. eCollection 2011. Front Microbiol. 2011. PMID: 21687427 Free PMC article. - Temporal and spatial trigger of post-exponential virulence-associated regulatory cascades by Legionella pneumophila after bacterial escape into the host cell cytosol.
Molmeret M, Jones S, Santic M, Habyarimana F, Esteban MT, Kwaik YA. Molmeret M, et al. Environ Microbiol. 2010 Mar;12(3):704-15. doi: 10.1111/j.1462-2920.2009.02114.x. Epub 2009 Dec 2. Environ Microbiol. 2010. PMID: 19958381 Free PMC article. - Replication of Legionella Pneumophila in Human Cells: Why are We Susceptible?
Khweek AA, Amer A. Khweek AA, et al. Front Microbiol. 2010 Dec 28;1:133. doi: 10.3389/fmicb.2010.00133. eCollection 2010. Front Microbiol. 2010. PMID: 21687775 Free PMC article. - Transcriptional down-regulation and rRNA cleavage in Dictyostelium discoideum mitochondria during Legionella pneumophila infection.
Zhang C, Kuspa A. Zhang C, et al. PLoS One. 2009 May 27;4(5):e5706. doi: 10.1371/journal.pone.0005706. PLoS One. 2009. PMID: 19492077 Free PMC article.
References
- Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–696. - PubMed
- Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. - PubMed
- Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources