Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation - PubMed (original) (raw)
Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation
G Hansen et al. J Clin Invest. 1999 Jan.
Abstract
Allergic asthma, which is present in as many as 10% of individuals in industrialized nations, is characterized by chronic airway inflammation and hyperreactivity induced by allergen-specific Th2 cells secreting interleukin-4 (IL-4) and IL-5. Because Th1 cells antagonize Th2 cell functions, it has been proposed that immune deviation toward Th1 can protect against asthma and allergies. Using an adoptive transfer system, we assessed the roles of Th1, Th2, and Th0 cells in a mouse model of asthma and examined the capacity of Th1 cells to counterbalance the proasthmatic effects of Th2 cells. Th1, Th2, and Th0 lines were generated from ovalbumin (OVA)-specific T-cell receptor (TCR) transgenic mice and transferred into lymphocyte-deficient, OVA-treated severe combined immunodeficiency (SCID) mice. OVA-specific Th2 and Th0 cells induced significant airway hyperreactivity and inflammation. Surprisingly, Th1 cells did not attenuate Th2 cell-induced airway hyperreactivity and inflammation in either SCID mice or in OVA-immunized immunocompetent BALB/c mice, but rather caused severe airway inflammation. These results indicate that antigen-specific Th1 cells may not protect or prevent Th2-mediated allergic disease, but rather may cause acute lung pathology. These findings have significant implications with regard to current therapeutic goals in asthma and allergy and suggest that conversion of Th2-dominated allergic inflammatory responses into Th1-dominated responses may lead to further problems.
Figures
Figure 1
Cytokine profiles of OVA-specific Th cell lines used in these studies. Th1, Th2, and Th0 cell lines (106 cells/ml) were stimulated with ConA (1 μg/ml) for 18 h. Supernatants were collected and analyzed by ELISA for IFN-γ, IL-4, and IL-5. IFN, interferon; IL, interleukin; OVA, ovalbumin.
Figure 2
OVA-TCR transgenic Th cells migrate to the lungs of recipient mice. Four days after adoptive transfer of OVA-specific Th cell lines (2.5 × 106 cells per mouse), mice were sacrificed and lung tissue was embedded in OCT compound. Frozen sections were obtained and stained with biotinylated anticlonotype antibody, KJ1-26.1 or biotinylated control antibody JES-312G8 and Streptavidin-FITC. (a) Lung tissue from recipient of Th1 cells. Left: Lung section stained with KJ1-26.1-biotin plus Streptavidin-FITC; Right: Lung section stained with biotinylated control antibody plus Streptavidin-FITC. (b) Lung tissue from recipient of Th2 cells. Left: Lung section stained with KJ1-26.1-biotin plus Streptavidin-FITC; Right: Lung section stained with biotinylated control antibody plus Streptavidin-FITC. TCR, T-cell receptor.
Figure 3
Histologic examination of lungs from SCID mice receiving Th1, Th2, and Th0 cells. (a) Lung tissue from control SCID mouse that received intranasal OVA but no Th cells. H&E, ×250. Inset: High-power magnification of normal bronchiolar epithelium. H&E, ×400. (b) Lung tissue from SCID mouse that received Th2 cells and intranasal OVA. Peribronchiolar mononuclear cell infiltrates are noted. The airway lumen is filled and expanded by thick mucus. H&E, ×250. Inset: High-power magnification of the airway epithelium showing tall columnar cells exhibiting abundant cytoplasmic mucin and a collarette of inflammatory cells. H&E, ×400. (c) Lung tissue from SCID mouse that received Th1 cells and intranasal OVA. Dense peribronchiolar inflammatory infiltrates are seen. The airway lumen does not contain mucus plugs. H&E, ×250. Inset: Lymphocytes are penetrating the airway epithelium and surrounding tissue spaces. H&E, ×400. (d) Lung tissue from control SCID mouse that received Th1 cells but not intranasal OVA. The bronchiole is normal with rare mononuclear cells in the peribronchiolar tissue; H&E ×250. Inset: The airway epithelium is normal. H&E, ×400. (e) Lung tissue from SCID mouse that received Th0 cells and intranasal OVA. Peribronchiolar infiltrates are noted, and the lumen is filled with mucus; scattered inflammatory cells are noted. H&E, ×250. Inset: The airway epithelium resembles that of mice that received OVA-specific Th2 cells, with the presence of tall columnar cells with abundant cytoplasmic mucin (b). (f) Lung tissue from SCID mouse that received both Th1 and Th2 cells and intranasal OVA. Significant airway inflammation is noted, without airway mucus. H&E, ×250. Inset: The epithelium displays reactive-appearing columnar cells, with inflammatory cells at the bases. H&E, ×400. H&E, hematoxylin and eosin; SCID, severe combined immunodeficiency.
Figure 4
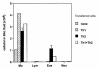
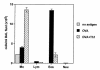
OVA-specific Th1 cells significantly reduce the number of eosinophils induced by OVA-specific Th2 cells in BAL fluid of OVA-treated SCID mice. Four days after transfer of Th1 (2.5 × 106 cells per mouse), Th2 (2.5 × 106 cells per mouse), or Th1 plus Th2 (2.5 × 106 Th1 + 2.5 × 106 Th2 cells per mouse) cells in OVA-treated SCID mice, BAL was performed with three aliquots of 0.4 ml PBS per mouse (n = 6 for each group). The relative number of different types of leukocytes (lung cell differentials) was determined from Hansel Stain slide preparations of BAL fluid. The data are expressed as mean ± SEM of the percentage of each cell type derived from differentials based on 200 cells. Results are given as cells per milliliter in BAL fluid. BAL, bronchoalveolar lavage; Eos, eosinophils; Lym, lymphocytes; Mo, macrophages; Neu, neutrophils.
Figure 5
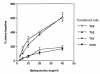
Th2 and Th0 cells, but not Th1 cells, increase airway hyperreactivity. SCID mice received Th1, Th2, or Th0 cells (2.5 × 106 cells per mouse) intravenously plus intranasal OVA. Control mice received either OVA only or cells only. Three days after adoptive cell transfer, airway hyperreactivity in response to increasing concentrations of inhaled methacholine was measured in a whole-body plethysmograph. Data are expressed as percent above baseline (mean ± SEM); n ≥ 7 for each data point. Cell transfer without intranasal administration of OVA had no effect on airway hyperreactivity (data not shown).
Figure 6
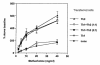
Th1 cells do not counterbalance airway hyperreactivity induced by Th2 cells. SCID mice received Th1 (2.5 × 106 cells per mouse) or Th2 (2.5 × 106 cells per mouse) cells intravenously plus intranasal OVA. Other SCID mice received a mixture of Th1 and Th2 cells in a ratio of 1:1 (2.5 × 106 cells each) or 2:1 (5 × 106 Th1 cells plus 2.5 × 106 Th2 cells) (n ≥ 5 for each data point). Airway hyperreactivity in response to inhaled methacholine was measured in a whole-body plethysmograph. Results are demonstrated as percent above baseline (mean ± SEM).
Figure 7
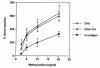
OVA-specific Th1 cells do not reduce airway hyperreactivity in OVA-immunized BALB/c mice. BALB/c mice were immunized with OVA (50 μg) in alum intraperitoneally on days 0 and 14, and intranasally (50 μg OVA in 50 μl PBS) on days 14, 25, 26, and 27 (OVA; n = 6). A second group of mice also received OVA-specific Th1 cells intravenously on days 14 and 25 (2.5 × 106 cells per mouse at each time point) (OVA + Th1; n = 5). Control mice received alum intraperitoneally and PBS intranasally (no antigen; n = 6). Airway hyperreactivity to methacholine was determined as in Figs. 5 and 6. Results are expressed as mean ± SEM. Transfer of Th1 cells without administration of antigen did not have any effect on airway hyperreactivity (data not shown).
Figure 8
OVA-specific Th1 cells significantly reduce the number of eosinophils in OVA-immunized BALB/c mice. BALB/c mice were immunized with OVA (50 μg) in alum intraperitoneally on days 0 and 14 and intranasally (50 μg OVA in 50 μl PBS) on days 14, 25, 26, and 27 (OVA; n = 6). A second group of mice was immunized with OVA and additionally received OVA-specific Th1 cells intravenously on days 14 and 25 (2.5 × 106 cells per mouse at each time point) (OVA + Th1; n = 5). Control mice received alum intraperitoneally and PBS intranasally (no antigen; n = 6). BAL was performed on day 29 with three aliquots of 0.4 ml PBS per mouse, and the relative number of different types of leukocytes (lung cell differentials) was determined. The data are expressed as mean ± SEM of the percentage of each cell type derived from differentials based on 200 cells.
Similar articles
- Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism.
Tang C, Inman MD, van Rooijen N, Yang P, Shen H, Matsumoto K, O'Byrne PM. Tang C, et al. J Immunol. 2001 Feb 1;166(3):1471-81. doi: 10.4049/jimmunol.166.3.1471. J Immunol. 2001. PMID: 11160186 - Zerumbone enhances the Th1 response and ameliorates ovalbumin-induced Th2 responses and airway inflammation in mice.
Shieh YH, Huang HM, Wang CC, Lee CC, Fan CK, Lee YL. Shieh YH, et al. Int Immunopharmacol. 2015 Feb;24(2):383-391. doi: 10.1016/j.intimp.2014.12.027. Epub 2015 Jan 5. Int Immunopharmacol. 2015. PMID: 25573403 - Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma.
Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW, Kemeny DM, Chung KF. Huang TJ, et al. J Immunol. 2001 Jan 1;166(1):207-17. doi: 10.4049/jimmunol.166.1.207. J Immunol. 2001. PMID: 11123294 - When engineered to produce latent TGF-beta1, antigen specific T cells down regulate Th1 cell-mediated autoimmune and Th2 cell-mediated allergic inflammatory processes.
Thorbecke GJ, Umetsu DT, deKruyff RH, Hansen G, Chen LZ, Hochwald GM. Thorbecke GJ, et al. Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):89-96. doi: 10.1016/s1359-6101(99)00032-5. Cytokine Growth Factor Rev. 2000. PMID: 10708956 Review. - Critical Involvement of CD44 in T Helper Type 2 Cell-Mediated Eosinophilic Airway Inflammation in a Mouse Model of Acute Asthma.
Katoh S. Katoh S. Front Immunol. 2022 Jan 7;12:811600. doi: 10.3389/fimmu.2021.811600. eCollection 2021. Front Immunol. 2022. PMID: 35069598 Free PMC article. Review.
Cited by
- Targeting aging and age-related diseases with vaccines.
Wu R, Sun F, Zhang W, Ren J, Liu GH. Wu R, et al. Nat Aging. 2024 Apr;4(4):464-482. doi: 10.1038/s43587-024-00597-0. Epub 2024 Apr 15. Nat Aging. 2024. PMID: 38622408 Review. - Gene-edited pigs: a translational model for human food allergy against alpha-Gal and anaphylaxis.
Wang Y, Hils M, Fischer A, Wölbing F, Biedermann T, Schnieke A, Fischer K. Wang Y, et al. Front Immunol. 2024 Feb 26;15:1358178. doi: 10.3389/fimmu.2024.1358178. eCollection 2024. Front Immunol. 2024. PMID: 38469303 Free PMC article. - Immunologic, genetic, and ecological interplay of factors involved in allergic diseases.
Falcon RMG, Caoili SEC. Falcon RMG, et al. Front Allergy. 2023 Aug 3;4:1215616. doi: 10.3389/falgy.2023.1215616. eCollection 2023. Front Allergy. 2023. PMID: 37601647 Free PMC article. Review. - Development of Adaptive Immunity and Its Role in Lung Remodeling.
Esnault S, Jarjour NN. Esnault S, et al. Adv Exp Med Biol. 2023;1426:287-351. doi: 10.1007/978-3-031-32259-4_14. Adv Exp Med Biol. 2023. PMID: 37464127
References
- Gergen PJ, Weiss KB. Changing patterns of asthma hospitalization among children: 1979 to 1987. JAMA. 1990;264:1688–1692. - PubMed
- Kirby JG, Hargreave FE, Gleich GJ, O’Byrne PM. Bronchoalveolar cell profiles of asthmatic and non-asthmatic subjects. Am Rev Respir Dis. 1987;136:379–383. - PubMed
- Bradley LB, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–674. - PubMed
- Umetsu DT, DeKruyff RH. Th1 and Th2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6. - PubMed
- Martinez FD, et al. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical