Detection of shiga-like toxin (stx1 and stx2), intimin (eaeA), and enterohemorrhagic Escherichia coli (EHEC) hemolysin (EHEC hlyA) genes in animal feces by multiplex PCR - PubMed (original) (raw)
Detection of shiga-like toxin (stx1 and stx2), intimin (eaeA), and enterohemorrhagic Escherichia coli (EHEC) hemolysin (EHEC hlyA) genes in animal feces by multiplex PCR
P K Fagan et al. Appl Environ Microbiol. 1999 Feb.
Abstract
A multiplex PCR was developed for the rapid detection of genes encoding Shiga toxins 1 and 2 (stx1 and stx2), intimin (eaeA), and enterohemolysin A (hlyA) in 444 fecal samples derived from healthy and clinically affected cattle, sheep, pigs, and goats. The method involved non-solvent-based extraction of nucleic acid from an aliquot of an overnight culture of feces in EC (modified) broth. The detection limit of the assay for both fecal samples and pure cultures was between 18 and 37 genome equivalents. stx1 and hlyA were the most commonly encountered virulence factors.
Figures
FIG. 1
Sensitivity of multiplex PCR in detecting EHEC virulence factors using serial dilutions of E. coli O111:H−. DNA markers are indicated on the right (numbers are molecular weights, in base pairs). Lanes 1 through 13 contain 6,250, 2,500, 630, 372, 186, 93, 46, 37, 18, 9, 3, 1.5, and <1 genome equivalents, respectively.
FIG. 2
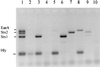
Multiplex PCR analysis of EHEC reference strains. Arrows A and B refer to nonspecific PCR bands (see the text). Lanes: 1, E. coli O111:H8; 2, E. coli O157:H7; 3, E. coli O128:H2; 4, E. coli O91:H−; 5, E. coli O113:H21; 6, E. coli O5:H−; 7, E. coli O111:H− positive control.
FIG. 3
Multiplex PCR analysis of bovine fecal samples. Lanes: 1, O111:H− positive control; 2 through 10, fecal samples collected on a farm previously identified as containing EHEC-positive animals.
Similar articles
- Semi-automated fluorogenic PCR assays (TaqMan) forrapid detection of Escherichia coli O157:H7 and other shiga toxigenic E. coli.
Sharma VK, Dean-Nystrom EA, Casey TA. Sharma VK, et al. Mol Cell Probes. 1999 Aug;13(4):291-302. doi: 10.1006/mcpr.1999.0251. Mol Cell Probes. 1999. PMID: 10441202 - Shiga-toxin-producing Escherichia coli in faeces of healthy dairy cows, sheep and goats: prevalence and virulence properties.
Zschöck M, Hamann HP, Kloppert B, Wolter W. Zschöck M, et al. Lett Appl Microbiol. 2000 Sep;31(3):203-8. doi: 10.1046/j.1365-2672.2000.00789.x. Lett Appl Microbiol. 2000. PMID: 10972729 - Detection of enterohemorrhagic Escherichia coli O157:H7 by using a multiplex real-time PCR assay for genes encoding intimin and Shiga toxins.
Sharma VK, Dean-Nystrom EA. Sharma VK, et al. Vet Microbiol. 2003 May 29;93(3):247-60. doi: 10.1016/s0378-1135(03)00039-7. Vet Microbiol. 2003. PMID: 12695048 - Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity.
Cavalieri SJ, Bohach GA, Snyder IS. Cavalieri SJ, et al. Microbiol Rev. 1984 Dec;48(4):326-43. doi: 10.1128/mr.48.4.326-343.1984. Microbiol Rev. 1984. PMID: 6394977 Free PMC article. Review. No abstract available.
Cited by
- Use of antibody responses against locus of enterocyte effacement (LEE)-encoded antigens to monitor enterohemorrhagic Escherichia coli infections on cattle farms.
Joris MA, Vanrompay D, Verstraete K, De Reu K, De Zutter L, Cox E. Joris MA, et al. Appl Environ Microbiol. 2013 Jun;79(12):3677-83. doi: 10.1128/AEM.01029-13. Epub 2013 Apr 5. Appl Environ Microbiol. 2013. PMID: 23563950 Free PMC article. - Association of Escherichia coli O157:H7 with houseflies on a cattle farm.
Alam MJ, Zurek L. Alam MJ, et al. Appl Environ Microbiol. 2004 Dec;70(12):7578-80. doi: 10.1128/AEM.70.12.7578-7580.2004. Appl Environ Microbiol. 2004. PMID: 15574966 Free PMC article. - Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host.
Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, Pearce MC, McKendrick IJ, Smith DG, Gally DL. Naylor SW, et al. Infect Immun. 2003 Mar;71(3):1505-12. doi: 10.1128/IAI.71.3.1505-1512.2003. Infect Immun. 2003. PMID: 12595469 Free PMC article. - A simple and rapid procedure for the detection of genes encoding Shiga toxins and other specific DNA sequences.
Nejman-Faleńczyk B, Bloch S, Januszkiewicz A, Węgrzyn A, Węgrzyn G. Nejman-Faleńczyk B, et al. Toxins (Basel). 2015 Nov 13;7(11):4745-57. doi: 10.3390/toxins7114745. Toxins (Basel). 2015. PMID: 26580652 Free PMC article. - Antimicrobial Resistance in Escherichia coli and Enterococcal Isolates From Irrigation Return Flows in a High-Desert Watershed.
Dungan RS, Bjorneberg DL. Dungan RS, et al. Front Microbiol. 2021 May 12;12:660697. doi: 10.3389/fmicb.2021.660697. eCollection 2021. Front Microbiol. 2021. PMID: 34054760 Free PMC article.
References
- Acheson D W, Keusch G T. Which Shiga toxin-producing types of Escherichia coli are important? ASM News. 1996;62:302–306.
- Agin T S, Cantey J R, Boedeker E C, Wolf M K. Characterization of the eaeA gene from rabbit enteropathogenic Escherichia coli strain RDEC-1 and comparison to other eaeA genes from bacteria that cause attaching-effacing lesions. FEMS Microbiol Lett. 1996;144:249–258. - PubMed
- Armstrong G L, Hollingsworth J, Morris J G. Emerging food borne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol Rev. 1996;18:29–50. - PubMed
- Bettelheim K A. Enterohaemorrhagic Escherichia coli—a review. Int Food Hyg. 1996;7:5–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical