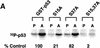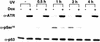A role for ATR in the DNA damage-induced phosphorylation of p53 - PubMed (original) (raw)
A role for ATR in the DNA damage-induced phosphorylation of p53
R S Tibbetts et al. Genes Dev. 1999.
Abstract
Phosphorylation at Ser-15 may be a critical event in the up-regulation and functional activation of p53 during cellular stress. In this report we provide evidence that the ATM-Rad3-related protein ATR regulates phosphorylation of Ser-15 in DNA-damaged cells. Overexpression of catalytically inactive ATR (ATRki) in human fibroblasts inhibited Ser-15 phosphorylation in response to gamma-irradiation and UV light. In gamma-irradiated cells, ATRki expression selectively interfered with late-phase Ser-15 phosphorylation, whereas ATRki blocked UV-induced Ser-15 phosphorylation in a time-independent manner. ATR phosphorylated p53 at Ser-15 and Ser-37 in vitro, suggesting that p53 is a target for phosphorylation by ATR in DNA-damaged cells.
Figures
Figure 1
Overexpression of ATRki inhibits DNA damage-induced phosphorylation of p53 on Ser-15. (A) Inhibition of γ-radiation-induced Ser-15 phosphorylation. GM847/ATRki cells were cultured either in the absence (−) or presence (+) of Dox for 48 hr to induce ATRki. Cells were either left untreated or exposed to 20 Gy of γ-radiation, and cell lysates were prepared after 4 hr. Immunoblots were performed with the indicated antibodies. (B) Time course of Ser-15 phosphorylation. Cells were treated as in A, except that cells were harvested at the indicated times after irradiation.
Figure 1
Overexpression of ATRki inhibits DNA damage-induced phosphorylation of p53 on Ser-15. (A) Inhibition of γ-radiation-induced Ser-15 phosphorylation. GM847/ATRki cells were cultured either in the absence (−) or presence (+) of Dox for 48 hr to induce ATRki. Cells were either left untreated or exposed to 20 Gy of γ-radiation, and cell lysates were prepared after 4 hr. Immunoblots were performed with the indicated antibodies. (B) Time course of Ser-15 phosphorylation. Cells were treated as in A, except that cells were harvested at the indicated times after irradiation.
Figure 2
ATR phosphorylates p53 on Ser-15 and Ser-37. (A) Wild-type GST–p53, and p53 mutants bearing single (S15A and S37A) or double (S15A, S37A) Ser → Ala amino acid substitutions were tested as ATR substrates. ATR was immunoprecipitated from testis extracts using either preimmune rabbit serum (P) or α-ATR (A). Numbers below each lane indicate the incorporation of [32P]phosphate into each substrate normalized to the value obtained with wild-type GST–p53. (B) Immunoreactivity of the phosphorylated wild-type and mutant GST–p53 fusion proteins with antibodies specific for phospho-Ser-15 and phospho-Ser-37, respectively. (C) Phosphorylation of p53 by ATR requires a functional kinase domain. K562 cells were transiently transfected with either pcDNA3, or plasmid constructs encoding Flag-tagged ATR (ATRwt), or Flag-tagged ATRki. Cell extracts were immunoprecipitated with Flag mAb, and immune complex kinase assays were performed with GST–p53 as the substrate. (D) ATR kinase activity in AT cells. Detergent extracts prepared from AT3BI cells or bovine testis extract were immunoprecipitated as in A, and kinase assays performed as described.
Figure 2
ATR phosphorylates p53 on Ser-15 and Ser-37. (A) Wild-type GST–p53, and p53 mutants bearing single (S15A and S37A) or double (S15A, S37A) Ser → Ala amino acid substitutions were tested as ATR substrates. ATR was immunoprecipitated from testis extracts using either preimmune rabbit serum (P) or α-ATR (A). Numbers below each lane indicate the incorporation of [32P]phosphate into each substrate normalized to the value obtained with wild-type GST–p53. (B) Immunoreactivity of the phosphorylated wild-type and mutant GST–p53 fusion proteins with antibodies specific for phospho-Ser-15 and phospho-Ser-37, respectively. (C) Phosphorylation of p53 by ATR requires a functional kinase domain. K562 cells were transiently transfected with either pcDNA3, or plasmid constructs encoding Flag-tagged ATR (ATRwt), or Flag-tagged ATRki. Cell extracts were immunoprecipitated with Flag mAb, and immune complex kinase assays were performed with GST–p53 as the substrate. (D) ATR kinase activity in AT cells. Detergent extracts prepared from AT3BI cells or bovine testis extract were immunoprecipitated as in A, and kinase assays performed as described.
Figure 2
ATR phosphorylates p53 on Ser-15 and Ser-37. (A) Wild-type GST–p53, and p53 mutants bearing single (S15A and S37A) or double (S15A, S37A) Ser → Ala amino acid substitutions were tested as ATR substrates. ATR was immunoprecipitated from testis extracts using either preimmune rabbit serum (P) or α-ATR (A). Numbers below each lane indicate the incorporation of [32P]phosphate into each substrate normalized to the value obtained with wild-type GST–p53. (B) Immunoreactivity of the phosphorylated wild-type and mutant GST–p53 fusion proteins with antibodies specific for phospho-Ser-15 and phospho-Ser-37, respectively. (C) Phosphorylation of p53 by ATR requires a functional kinase domain. K562 cells were transiently transfected with either pcDNA3, or plasmid constructs encoding Flag-tagged ATR (ATRwt), or Flag-tagged ATRki. Cell extracts were immunoprecipitated with Flag mAb, and immune complex kinase assays were performed with GST–p53 as the substrate. (D) ATR kinase activity in AT cells. Detergent extracts prepared from AT3BI cells or bovine testis extract were immunoprecipitated as in A, and kinase assays performed as described.
Figure 2
ATR phosphorylates p53 on Ser-15 and Ser-37. (A) Wild-type GST–p53, and p53 mutants bearing single (S15A and S37A) or double (S15A, S37A) Ser → Ala amino acid substitutions were tested as ATR substrates. ATR was immunoprecipitated from testis extracts using either preimmune rabbit serum (P) or α-ATR (A). Numbers below each lane indicate the incorporation of [32P]phosphate into each substrate normalized to the value obtained with wild-type GST–p53. (B) Immunoreactivity of the phosphorylated wild-type and mutant GST–p53 fusion proteins with antibodies specific for phospho-Ser-15 and phospho-Ser-37, respectively. (C) Phosphorylation of p53 by ATR requires a functional kinase domain. K562 cells were transiently transfected with either pcDNA3, or plasmid constructs encoding Flag-tagged ATR (ATRwt), or Flag-tagged ATRki. Cell extracts were immunoprecipitated with Flag mAb, and immune complex kinase assays were performed with GST–p53 as the substrate. (D) ATR kinase activity in AT cells. Detergent extracts prepared from AT3BI cells or bovine testis extract were immunoprecipitated as in A, and kinase assays performed as described.
Figure 3
γ-Radiation-induced ATM activation in ATRki overexpressing cells. GM847/ATRki cells were cultured and cells treated as in Fig. 1A. After 1 hr, ATM was immunoprecipitated and ATM kinase assays were performed as in Fig. 2A. Numbers below sample lanes are as in Fig. 2A. Levels of ATM protein were determined by immunoblotting with α-ATM (bottom).
Figure 4
Effects of ATRki overexpression on Ser-15 phosphorylation induced by UV light. GM847/ATRki cells were cultured as in Fig. 1A and were either left untreated or exposed to 50 J/m2 UV light; cell lysates were prepared at the indicated time intervals. Detergent-soluble proteins were separated by SDS-PAGE and were immunoblotted with the indicated antibodies.
References
- Anderson CW, Carter TH. The DNA-activated protein kinase–DNA-PK. Curr Top Microbiol Immunol. 1996;217:91–111. - PubMed
- Banin S, Moyal L, Shieh SY, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
- Beamish H, Williams R, Chen P, Lavin MF. Defect in multiple cell cycle checkpoints in ataxia-telangiectasia postirradiation. J Biol Chem. 1996;271:20486–20493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA052995/CA/NCI NIH HHS/United States
- T32 CA009441/CA/NCI NIH HHS/United States
- U19 CA052995/CA/NCI NIH HHS/United States
- CA58316/CA/NCI NIH HHS/United States
- R01 CA058316/CA/NCI NIH HHS/United States
- CA76193/CA/NCI NIH HHS/United States
- R37 CA058316/CA/NCI NIH HHS/United States
- CA52995/CA/NCI NIH HHS/United States
- R01 CA076193/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous