Age-dependent, steroid-specific effects of oestrogen on long-term potentiation in rat hippocampal slices - PubMed (original) (raw)
Age-dependent, steroid-specific effects of oestrogen on long-term potentiation in rat hippocampal slices
K Ito et al. J Physiol. 1999.
Abstract
1. Long-term potentiation (LTP) of hippocampal population spike responses and excitatory postsynaptic potentials (EPSPs) from area CA1 stratum pyramidale was induced in slices of rat hippocampus maintained in vitro following brief high-frequency stimulation (HFS) of the Schaffer collateral-commissural pathway. When administered to slices prior to HFS, 17beta-oestradiol (OE2), at a concentration as low as 0.1 nM, suppressed the magnitude of the resultant HFS-induced potentiation in slices from prepubertal animals (3 and 4 weeks old) of both sexes. 2. OE2 did not suppress the induction of LTP in slices taken from the hippocampus of adult animals of either sex. 3. There was no similar suppressant effect of 17alpha-oestradiol (OE1), progesterone (PRG) or testosterone (TST) on LTP in the young animals, even at a concentration 100 times greater than was effective for OE2. 4. The anti-oestrogen compound tamoxifen (TMX; 1.0 and 10.0 microM), which acts principally at intracellular binding sites within the nucleus, was without effect in diminishing the suppressant effect of OE2 on LTP in slices from young animals. 5. The LTP observed in slices from both 3-week-old and adult rats was AP5 sensitive and thus was shown to be dependent on activation of NMDA receptors. Results from whole-cell recording experiments suggested that OE2 caused the LTP-suppressant effect through an action on NMDA-mediated currents. 6. These data suggest an age-dependent and possibly a surface membrane receptor-mediated role for oestrogens in modulating the efficacy of input-output properties of CA1 neurones produced by HFS during a critical period in development.
Figures
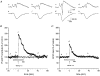
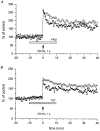
Figure 1. Data from a single experiment, using a hippocampal slice from a 4-week-old female in the presence of 10 nM OE2 during administration of HFS
Aa-d, population spike recordings; Ae-h, field EPSP slopes showing sample recordings taken prior to (a and b, and e and f) and after (c and d, and g and h) HFS, at times corresponding to similarly lettered points on the graphs in B and C. Each circle in B, and triangle in C, shows population spike (PS) and fibre volley (FV) amplitude data, and EPSP slope data, respectively, recorded during each minute of a trial, representing the computer-calculated means of 6 successively sampled sweeps. Crosses in B represent amplitude of FV. Hatched bar below graph indicates period of time during which OE2 was administered.
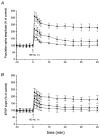
Figure 2. Mean effects of OE2 (0.1 nM and 10 nM) on elicitation of LTP from area CA1 in slices from 4-week-old rats
HFS was delivered at t = 0. Amplitudes of the population spike responses (A) and field EPSPs (B) are plotted against the ordinate scale where 100 % refers to the value of the response prior to HFS, irrespective of the test condition (▪, drug free; •, 0.1 nM OE2; and ▴, 10 nM OE2). Points in each of the 3 conditions represent means of 12 observations.
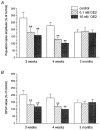
Figure 3
Summary of the effects of HFS on the magnitude of the increase of CA1 population spike responses (A) and field EPSPs (B) in the drug-free condition (open bars) and in the presence of OE2 (hatched bars: 0.1 nM; shaded bars: 10 nM), in the hippocampus of 3-week-old (n = 10), 4-week-old (n = 12) and 3-month-old (n = 9) rats. Results with males and females in all categories pooled. Error bars in this and all other figures represent ± 1
s.d.
** Data that differ significantly (P < 0.01) from the controls in the corresponding age category.
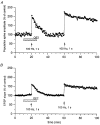
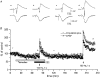
Figure 4. Effect of OE2 on LTP in a slice taken from the hippocampus of a 4-week-old male
A, population spike data; B, field EPSP data. Two series of HFS were delivered to the same slice, the 1st in the presence of 10 nM OE2, and the 2nd 40 min later (33 min after removal of OE2 from the perfusing medium). For all other conventions, see legend to Fig. 1.
Figure 5
Examples of the results of tests with PRG (A) and TST (B), both delivered at 10 nM, on LTP in slices from 4-week-old rats. Each substance was administered 10 min prior to HFS and was removed from the perfusing medium 10 min following cessation of stimulation. ○, population spike data; ▴, field EPSP data.
Figure 6
A, sample synaptic traces and, B, graphical representation of data from a single experiment illustrating lack of antagonism by TMX (administered during period shown by hatched bar) of the OE2-induced (filled bar) suppression of LTP in area CA1. a, drug-free condition; b, 60 min after administration of TMX (1.0 μM); c, 40 min after HFS in the presence of OE2 and TMX and 25 min following drug removal; d, 30 min after a second HFS delivered during drug-free condition. In A, top, sample traces of population spike data; A, bottom, field EPSP traces. For all other conventions, see legend to Fig. 1.
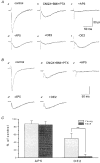
Figure 7. The effect of OE2 on synaptic responses recorded in whole-cell mode showing the component mediated through NMDA receptors in two neurones
A, from the 3-week-old group. B, from the 3-month-old group. Aa, control response. Ab, in the presence of CNQX (10 μM), BMI (10 μM) and PTX (10 μM) (CBP solution). Ac, in the presence of the same drugs as Ab but with AP5 (50 μM) added. Ad, the response in CBP solution after removal of AP5. Ae, after addition of OE2 (10 nM). Af, recovery after removal of OE2 in CBP solution. Ba'-e', same conventions as for Aa-f, but recordings from a neurone in an adult. Vertical bar is 60 pA for a' and 30 pA for b'-e'. C, bar histograms showing data relating only to the isolated, AP5-sensitive component of the synaptically elicited responses of young and adult CA1 neurones. Left pair of bars represent this component in young (3 weeks, hatched column, n = 8) and adult animals (3 months, shaded column, n = 8). Right pair of bars represent the OE2-sensitive component in the two age groups, as indicated. Note the much greater OE2-sensitive component in the responses from the young neurones. ** Statistically significant (Student's t test, P < 0.01).
Similar articles
- 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation.
Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. Foy MR, et al. J Neurophysiol. 1999 Feb;81(2):925-9. doi: 10.1152/jn.1999.81.2.925. J Neurophysiol. 1999. PMID: 10036289 - Short-term facilitation evoked during brief afferent tetani is not altered by long-term potentiation in the guinea-pig hippocampal CA1 region.
Pananceau M, Chen H, Gustafsson B. Pananceau M, et al. J Physiol. 1998 Apr 15;508 ( Pt 2)(Pt 2):503-14. doi: 10.1111/j.1469-7793.1998.503bq.x. J Physiol. 1998. PMID: 9508813 Free PMC article. - Involvement of excitatory amino acid receptors in long-term potentiation in the Schaffer collateral-commissural pathway of rat hippocampal slices.
Collingridge GL, Blake JF, Brown MW, Bashir ZI, Ryan E. Collingridge GL, et al. Can J Physiol Pharmacol. 1991 Jul;69(7):1084-90. doi: 10.1139/y91-160. Can J Physiol Pharmacol. 1991. PMID: 1659488 Review. - Pharmacological study on Alzheimer's drugs targeting calcium/calmodulin-dependent protein kinase II.
Moriguchi S. Moriguchi S. J Pharmacol Sci. 2011;117(1):6-11. doi: 10.1254/jphs.11r06cp. Epub 2011 Aug 6. J Pharmacol Sci. 2011. PMID: 21821968 Review.
Cited by
- Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity.
Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hojo Y, et al. Front Endocrinol (Lausanne). 2011 Oct 10;2:43. doi: 10.3389/fendo.2011.00043. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22701110 Free PMC article. - The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus.
Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. Bi R, et al. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3602-7. doi: 10.1073/pnas.97.7.3602. Proc Natl Acad Sci U S A. 2000. PMID: 10725383 Free PMC article. - Progesterone regulation of synaptic transmission and plasticity in rodent hippocampus.
Foy MR, Akopian G, Thompson RF. Foy MR, et al. Learn Mem. 2008 Oct 30;15(11):820-2. doi: 10.1101/lm.1124708. Print 2008 Nov. Learn Mem. 2008. PMID: 18984562 Free PMC article. - Synaptic Plasticity Abnormalities in Fetal Alcohol Spectrum Disorders.
Basavarajappa BS, Subbanna S. Basavarajappa BS, et al. Cells. 2023 Jan 29;12(3):442. doi: 10.3390/cells12030442. Cells. 2023. PMID: 36766783 Free PMC article. Review. - N-cadherin is regulated by gonadal steroids in the adult hippocampus.
Monks DA, Getsios S, MacCalman CD, Watson NV. Monks DA, et al. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1312-6. doi: 10.1073/pnas.98.3.1312. Epub 2001 Jan 23. Proc Natl Acad Sci U S A. 2001. PMID: 11158636 Free PMC article.
References
- Ball J. The female sex cycle as a factor in learning in the rat. American Journal of Physiology. 1926;78:533–536.
- Collingridge GL, Bliss TVP. NMDA receptors - their role in long-term potentiation. Trends in Neurosciences. 1987;10:288–293.
- Döcke F, Rohde W, Smollich A, Dörner G. Hormones and brain maturation in the control of female puberty. In: Dörner G, Kawakami M, editors. Hormones and Brain Development. Amsterdam: Elsevier/North Holland Biomedical Press; 1978. pp. 327–340.
- Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin, and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous