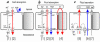The genesis of cystic fibrosis lung disease - PubMed (original) (raw)
Review
The genesis of cystic fibrosis lung disease
J J Wine. J Clin Invest. 1999 Feb.
No abstract available
Figures
Figure 1
CFTR's multiple roles in fluid and electrolyte transport. (a) Salt absorption. In the sweat duct, high apical conductance for Na+ [1] and Cl– [2] and relatively low water conductance allows salt to be reabsorbed in excess of water (hypertonic absorption) leaving a hypotonic luminal fluid. In the sweat duct CFTR is the only available anion conductance pathway, and when it is lost in CF the lumen quickly becomes highly electronegative and transport virtually ceases, resulting in high (similar to plasma) luminal salt (b). Fluid absorption. In epithelia with high water permeability [3] relative to electrolyte permeability water will absorbed osmotically with salt to decrease the volume of luminal fluid. If no other osmolytes or forces are present, the salt concentration will remain unchanged. If water-retaining forces are present, permeant electrolytes can be reduced preferentially. The consequences of eliminating CFTR depend on the magnitude of such forces, the relative magnitude of alternate pathways for transepithelial anion flow [4], and how CFTR affects other ion channels. The high salt and low volume hypotheses differ on each of these points. (c) Anion-mediated fluid secretion. Secreting epithelia lack a significant apical Na+ conductance. Basolateral transporters such as NKCC move Cl– uphill into the cell; it then flows passively into the lumen via CFTR [5], K+ exits basolaterally, Na+ flows paracellularly [7] and water follows transcellularly [6]. Elimination of CFTR eliminates secretion.
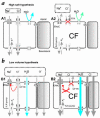
Figure 2
Two hypotheses of how airway surface liquid (ASL) differs in healthy and CF lungs. (a) The high salt hypothesis (9, 10) postulates that normal ASL has low levels of salt as a result of salt absorption in excess of water (A1, left). Even though the epithelium is water permeable, salt is retained in thin surface films by some combination of surface tension (28) and impermeant osmolytes (10). In CF (A2), salt is poorly absorbed resulting in excessively salty ASL that disrupts natural mucosal antibiotics. Key features of the high salt model are: the lack of an appreciable shunt Cl– conductance, central importance of CFTR's channel role, no specific role for inhibition of ENaC by CFTR, and a switch from isotonic volume absorption to hypertonic salt absorption as the surface layer thins and traps residual water. (b) The low volume hypothesis (14) postulates that normal ASL (B1) has salt levels approximately equal to plasma. In CF (B2), the removal of CFTR's inhibition of ENaC results in abnormally elevated isotonic fluid absorption which depletes the ASL and leads to reduced mucociliary clearance. Key features of the low volume model are the parallel pathway for Cl– via shunt pathway(s) and inhibition of ENaC via CFTR.
Comment in
- Status of gene therapy for cystic fibrosis lung disease.
Boucher RC. Boucher RC. J Clin Invest. 1999 Feb;103(4):441-5. doi: 10.1172/JCI6330. J Clin Invest. 1999. PMID: 10021450 Free PMC article. Review. No abstract available.
Similar articles
- The innate immune system in cystic fibrosis lung disease.
Bals R, Weiner DJ, Wilson JM. Bals R, et al. J Clin Invest. 1999 Feb;103(3):303-7. doi: 10.1172/JCI6277. J Clin Invest. 1999. PMID: 9927489 Free PMC article. Review. No abstract available. - A powerful method for in vitro selection of normal versus cystic fibrosis airway epithelial cells.
Vega MA, Goossens M, Besmond C. Vega MA, et al. Gene Ther. 1994 Jan;1(1):59-63. Gene Ther. 1994. PMID: 7584061 - Cystic fibrosis: a disease of vulnerability to airway surface dehydration.
Boucher RC. Boucher RC. Trends Mol Med. 2007 Jun;13(6):231-40. doi: 10.1016/j.molmed.2007.05.001. Epub 2007 May 23. Trends Mol Med. 2007. PMID: 17524805 - Innate immune activation and cystic fibrosis.
Brennan S. Brennan S. Paediatr Respir Rev. 2008 Dec;9(4):271-9; quiz 279-80. doi: 10.1016/j.prrv.2008.05.008. Epub 2008 Oct 15. Paediatr Respir Rev. 2008. PMID: 19026368 Review. - Expression of delta F508 cystic fibrosis transmembrane conductance regulator protein and related chloride transport properties in the gallbladder epithelium from cystic fibrosis patients.
Dray-Charier N, Paul A, Scoazec JY, Veissière D, Mergey M, Capeau J, Soubrane O, Housset C. Dray-Charier N, et al. Hepatology. 1999 Jun;29(6):1624-34. doi: 10.1002/hep.510290634. Hepatology. 1999. PMID: 10347100
Cited by
- The Evolution of Cystic Fibrosis Care.
Pittman JE, Ferkol TW. Pittman JE, et al. Chest. 2015 Aug;148(2):533-542. doi: 10.1378/chest.14-1997. Chest. 2015. PMID: 25764168 Free PMC article. Review. - Regulation of airway tight junctions by proinflammatory cytokines.
Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Coyne CB, et al. Mol Biol Cell. 2002 Sep;13(9):3218-34. doi: 10.1091/mbc.e02-03-0134. Mol Biol Cell. 2002. PMID: 12221127 Free PMC article. - Impact of mucus modulation by _N_-acetylcysteine on nanoparticle toxicity.
Meziu E, Shehu K, Koch M, Schneider M, Kraegeloh A. Meziu E, et al. Int J Pharm X. 2023 Sep 21;6:100212. doi: 10.1016/j.ijpx.2023.100212. eCollection 2023 Dec 15. Int J Pharm X. 2023. PMID: 37771516 Free PMC article. - Extracellular barriers in respiratory gene therapy.
Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J. Sanders N, et al. Adv Drug Deliv Rev. 2009 Feb 27;61(2):115-27. doi: 10.1016/j.addr.2008.09.011. Epub 2008 Dec 24. Adv Drug Deliv Rev. 2009. PMID: 19146894 Free PMC article. Review. - Front-runners for pharmacotherapeutic correction of the airway ion transport defect in cystic fibrosis.
Clunes MT, Boucher RC. Clunes MT, et al. Curr Opin Pharmacol. 2008 Jun;8(3):292-9. doi: 10.1016/j.coph.2008.04.006. Epub 2008 May 28. Curr Opin Pharmacol. 2008. PMID: 18468487 Free PMC article. Review.
References
- Egan M, et al. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992;358:581–584. - PubMed
- Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272:14037–14040. - PubMed
- Knowles MR, et al. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. - PubMed