Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization - PubMed (original) (raw)
Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization
M D Gunn et al. J Exp Med. 1999.
Abstract
Secondary lymphoid organ chemokine (SLC) is expressed in high endothelial venules and in T cell zones of spleen and lymph nodes (LNs) and strongly attracts naive T cells. In mice homozygous for the paucity of lymph node T cell (plt) mutation, naive T cells fail to home to LNs or the lymphoid regions of spleen. Here we demonstrate that expression of SLC is undetectable in plt mice. In addition to the defect in T cell homing, we demonstrate that dendritic cells (DCs) fail to accumulate in spleen and LN T cell zones of plt mice. DC migration to LNs after contact sensitization is also substantially reduced. The physiologic significance of these abnormalities in plt mice is indicated by a markedly increased sensitivity to infection with murine hepatitis virus. The plt mutation maps to the SLC locus; however, the sequence of SLC introns and exons in plt mice is normal. These findings suggest that the abnormalities in plt mice are due to a genetic defect in the expression of SLC and that SLC mediates the entry of naive T cells and antigen-stimulated DCs into the T cell zones of secondary lymphoid organs.
Figures
Figure 1
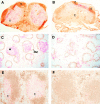
SLC mRNA is not expressed in plt mice. Sections of paraffin-embedded tissues from +/+ (A, C, and E) and plt (B, D, and F) mice were hybridized with 35S-labeled SLC antisense riboprobe and exposed for 8 wk. SLC hybridization signal (white dots) can be seen in LN (A), spleen (C), and PP (E) of +/+ mice. No SLC signal is detected in the LN (B), spleen (D), and PP (F) of plt mice. f, lymphoid follicles; t, T cell zone; RP, red pulp. (G) Total RNA from peripheral LNs (PLN) and spleen of +/+ and plt mice was subjected to Northern blot analysis with 32P-labeled SLC probe and subjected to autoradiography. Blots were stripped and reprobed with actin probe to determine variability in gel loading.
Figure 2
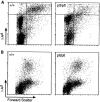
(A) The number of DCs is decreased in LNs of plt mice. LNs of +/+ and plt mice were collected and centrifuged over metrizamide gradients. Cells at the interface were collected, stained with FITC-conjugated anti–mouse I-Ad, normalized to the total number of cells per LN, and analyzed by FACS® to detect I-A+ DCs (top right quadrant). One representative experiment of three is shown. (B) The number of DCs is normal in spleens of plt mice. Spleens of +/+ and plt mice were collected, dissociated with collagenase, and stained with FITC-anti–I-Ad, biotinylated anti-B220, and biotinylated anti-CD3 followed by SA-PerCP, normalized to the total number of cells per spleen, and analyzed by flow cytometry. CD3−, B220− gated cells in one representative experiment of three are shown.
Figure 3
DCs do not accumulate in the T cell zones of plt mice. The distribution of DCs was examined in cryostat sections of +/+ (A, C, and E) and plt (B, D, and F) mice by immunohistochemistry. In LNs from +/+ mice (A), NLDC-145+ DCs (red) are distributed uniformly throughout the T cell zone (t). IgMa + B cells (brown) are shown for orientation. When LNs from plt mice are stained similarly (B), DCs are found only in the outer cortex between B cell follicles (f). In spleens of +/+ mice (C), numerous CD11c+ DCs (red) are seen within the white pulp (w). Marginal zones, which form the outer boundary of the white pulp, are identified by MOMA-1 staining (brown). In spleens of plt mice (D), few DCs appear within the white pulp. In spleens of +/+ mice (E), NLDC-145+ DCs (red) are seen within the T cell zone (t). IgMa + B cells (brown) are shown for orientation. In plt spleen (F), few NLDC-145+ cells are seen.
Figure 4
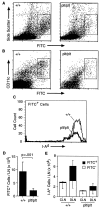
Decreased migration of skin DCs to LNs in plt mice after contact sensitization with FITC. (A) The shaved abdomens of +/+ and plt mice were painted with 2 mg FITC. After 24 h draining LNs were removed, dissociated, normalized to the total number of cells per LN, and analyzed by flow cytometry. A decreased number of large FITC+ cells (boxed area) can be seen in plt mice. One of eight representative experiments is shown. (B) Representative FACS® profile showing a marked decrease of CD11c+ FITC+ cells in plt mice after FITC skin painting. (C) Draining LN cells from FITC-painted mice were partially purified on metrizamide gradients, stained with biotinylated anti–I-Ad followed by SA-PerCP, and analyzed by flow cytometry. Only large FITC+ cells (boxed areas in A) are shown. (D) The number of FITC+ DCs (boxed areas in A) that accumulate in LNs after skin painting with 2 mg FITC is reduced in plt mice. Numbers represent mean ± SD (n = 8). (E) Comparison of DC content in contralateral (CLN) and draining (DLN) inguinal LNs in mice painted on one flank with 0.2 mg FITC. Single cell suspensions were prepared from individual LNs, stained with anti–I-Ad and anti-B220, and analyzed by flow cytometry gated on I-A+ B220− cells.
Figure 5
(A and B) Whole mounts of abdominal epidermis from +/+ (A) and plt (B) mice show normal numbers and distribution of DCs. Abdominal epidermis was separated from dermis and stained with anti–I-Ad (reference 31). (C and D) The accumulation of activated DCs in splenic T cell zones is decreased in plt mice. Spleens of +/+ (C) and plt (D) mice were removed 6 h after intraperitoneal injection of LPS. Frozen sections were prepared and stained for CD11c+ DCs (red) and B220+ B cells (brown).
Figure 6
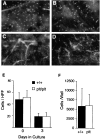
Migration of DCs out of skin explants is normal in plt mice. Dorsal ear skin was floated on medium and cultured (reference 31). After 72 h, dermal and epidermal whole mounts were prepared and stained with anti–I-Ad followed by SA-FITC. The density of DCs is similar in the epidermis of +/+ (A) and plt (B) mice after 72 h of culture. DC cords form within dermal lymphatics of both +/+ (C) and plt (D) mice after 72 h of culture. (E) The density of DCs in epidermis decreases similarly in +/+ and plt mice over 72 h of culture. I-A+ cells were counted in 20 fields/slide over 4 slides and calculated as the mean ± SD of cells/ HPF. (F) Emigration of DCs out of cultured skin is normal in plt mice. Nonadherent cells were collected from the bottom of wells in which ear skin had been cultured for 72 h. Total cell number was determined by counting on a hemocytometer. The proportion of DCs was calculated by examining anti–I-Ad–stained cytospins of nonadherent cells. Results represent the mean ± SD of DCs/well over four wells.
Figure 7
Expression of ELC mRNA is decreased in the LNs and spleens of plt mice. Tissues from +/+ (A and C) and plt (B and D) mice were analyzed as described in the legend to Fig. 1. ELC hybridization signal (white dots) can be seen in LN (A) and spleen (C) of +/+ mice. The intensity of ELC signal is reduced in the LN (B) and spleen (D) of plt mice. f, lymphoid follicles; RP, red pulp. (E) Northern blot analysis. Total RNA from peripheral LNs (PLN) and spleen of +/+ and plt mice was hybridized with 32P-labeled ELC probe and subjected to autoradiography. Blots were stripped and reprobed with actin probe to determine variability in gel loading.
Figure 8
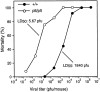
Increased sensitivity of plt mice to MHV infection. Mice were injected intraperitoneally with the indicated doses of MHV and scored for mortality over the next 2 wk. Each data point represents at least eight infected mice. The calculated LD50 for MHV infection of +/+ and plt mice are indicated.
Comment in
- Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs.
Cyster JG. Cyster JG. J Exp Med. 1999 Feb 1;189(3):447-50. doi: 10.1084/jem.189.3.447. J Exp Med. 1999. PMID: 9927506 Free PMC article. No abstract available.
Similar articles
- The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7.
Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. Willimann K, et al. Eur J Immunol. 1998 Jun;28(6):2025-34. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. Eur J Immunol. 1998. PMID: 9645384 - Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses.
Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mori S, et al. J Exp Med. 2001 Jan 15;193(2):207-18. doi: 10.1084/jem.193.2.207. J Exp Med. 2001. PMID: 11148224 Free PMC article. - Leukocyte migration: scent of the T zone.
Cyster JG. Cyster JG. Curr Biol. 2000 Jan 13;10(1):R30-3. doi: 10.1016/s0960-9822(99)00253-5. Curr Biol. 2000. PMID: 10660291 Review. - A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system.
Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. Comerford I, et al. Cytokine Growth Factor Rev. 2013 Jun;24(3):269-83. doi: 10.1016/j.cytogfr.2013.03.001. Epub 2013 Apr 12. Cytokine Growth Factor Rev. 2013. PMID: 23587803 Review.
Cited by
- The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice.
Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. Lefebvre JS, et al. Aging Cell. 2012 Oct;11(5):732-40. doi: 10.1111/j.1474-9726.2012.00836.x. Epub 2012 Jun 11. Aging Cell. 2012. PMID: 22607653 Free PMC article. - Sublingual Immunotherapy Induces Regulatory Function of IL-10-Expressing CD4(+)CD25(+)Foxp3(+) T Cells of Cervical Lymph Nodes in Murine Allergic Rhinitis Model.
Yamada T, Tongu M, Goda K, Aoi N, Morikura I, Fuchiwaki T, Kawauchi H. Yamada T, et al. J Allergy (Cairo). 2012;2012:490905. doi: 10.1155/2012/490905. Epub 2012 Oct 17. J Allergy (Cairo). 2012. PMID: 23118775 Free PMC article. - Increased chemokine (C-C motif) ligand 21 expression and its correlation with osteopontin in Graves' disease.
Qi Y, Li X, Zhang Q, Huang F, Lin D, Zhou Y, Hong J, Cui B, Wang W, Ning G, Wang S. Qi Y, et al. Endocrine. 2015 Sep;50(1):123-9. doi: 10.1007/s12020-015-0552-7. Epub 2015 Mar 15. Endocrine. 2015. PMID: 25771884 - Mature monocyte-derived dendritic cells respond more strongly to CCL19 than to CXCL12: consequences for directional migration.
Humrich JY, Humrich JH, Averbeck M, Thumann P, Termeer C, Kämpgen E, Schuler G, Jenne L. Humrich JY, et al. Immunology. 2006 Feb;117(2):238-47. doi: 10.1111/j.1365-2567.2005.02292.x. Immunology. 2006. PMID: 16423060 Free PMC article. - Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection.
Kursar M, Höpken UE, Koch M, Köhler A, Lipp M, Kaufmann SH, Mittrücker HW. Kursar M, et al. J Exp Med. 2005 May 2;201(9):1447-57. doi: 10.1084/jem.20041204. Epub 2005 Apr 25. J Exp Med. 2005. PMID: 15851484 Free PMC article.
References
- Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. - PubMed
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
- Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukocyte Biol. 1997;62:634–644. - PubMed
- Goodnow CC, Cyster JG. Lymphocyte homing: the scent of a follicle. Curr Biol. 1997;7:R219–R222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases