The stretch-activation response may be critical to the proper functioning of the mammalian heart - PubMed (original) (raw)
The stretch-activation response may be critical to the proper functioning of the mammalian heart
R Vemuri et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The "stretch-activation" response is essential to the generation of the oscillatory power required for the beating of insect wings. It has been conjectured but not previously shown that a stretch-activation response contributes to the performance of a beating heart. Here, we generated transgenic mice that express a human mutant myosin essential light chain derived from a family with an inherited cardiac hypertrophy. These mice faithfully replicate the cardiac disease of the patients with this mutant allele. They provide the opportunity to study the stretch-activation response before the hearts are distorted by the hypertrophic process. Studies disclose a mismatch between the physiologic heart rate and resonant frequency of the cardiac papillary muscles expressing the mutant essential light chain. This discordance reduces oscillatory power at frequencies that correspond to physiologic heart-rates and is followed by subsequent hypertrophy. It appears, therefore, that the stretch-activation response, first described in insect flight muscle, may play a role in the mammalian heart, and its further study may suggest a new way to modulate human cardiac function.
Figures
Figure 1
Representative gross anatomy, histology, and immunohistology of normal mouse hearts compared with hearts from transgenic mice expressing the normal human ELC (human control) or mutant human ELC (mutant human) at 3 months and at 1 year of age. (A_–_C) Hearts from normal (A), human control (B), and human mutant (C) mice, respectively, at 3 months of age are similar. (D_–_F) The morphology in H- & E-stained sections of the three hearts shown in A_–_C is normal. (×50.) (G_–_I) Immunoreactivity after staining with polyclonal antibody against mutant ELC is absent in normal hearts (G), minimal in human control hearts (H), and strong in the human mutant hearts (I) HR peroxidase method was used. (×50.) (I, Inset) Localization of reactivity in myofibrills by using a fluorescein isothiocyanate-conjugated (green) secondary antibody. (×150.) (J_–_L) Hearts from three different normal (J), human control (K), and human mutant (L) mice at 1 year. Solid arrows in J and K show normal left ventricular cavity. Arrow in L shows hypertrophied papillary muscle obstructing the middle of the mostly obliterated left ventricular cavity. (M_–_O) H- and E-stained sections of normal (M), human control (N), and human mutant (O) hearts at 1 year. M and N are normal; O shows myocyte hypertrophy without disarray. (×50.)
Figure 2
Tension as a function of time after a step increase of 1% in length of right ventricular papillary muscles from 3- to 4-month-old mice. The average of tension transients from muscles of three normal mice (normal), three transgenic mice expressing the normal human ELC (human control), and four transgenic mice expressing the mutant human ELC (human mutant) are as noted in the key. Three- to four-month-old mice were used to avoid hypertrophy in the human mutant mice. The corresponding vertical arrows in each case mark the peak of the “second rise in tension.” Just below the time axis are shown the corresponding time delays (tds) with time origin at 0 s from the initiation of stretch. The three traces show that the td for the muscles from the human mutant mice is ≈1/3 that for the normal and human control mice.
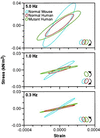
Figure 3
Smoothed work loops from raw data of fiber stress versus strain (normalized tension vs. fractional change in length) during sinusoidal length oscillations of representative normal, control human, and mutant mouse papillary muscles at three frequencies. Raw saved data was digitally filtered at 16× the driving frequency to reduce electrical noise (primarily 60 Hz). Data is shown at each frequency for the three types of muscle bundles. The muscle bundle type and direction of the work loop are identified by color and arrows respectively, in the legend. In the top panel are loops during 5.0 Hz oscillations showing that, at this frequency, all muscle bundles absorb work from the driving apparatus. The clockwise rotation of the loops indicates that, during shortening, the force exerted is lower than during lengthening. In the middle panel, at 1.0 Hz, only the mutant human bundle performs work on the driving apparatus, as indicated by the counter-clockwise rotation. In the bottom panel, at 0.3 Hz, all three bundles traverse a counter-clockwise loop, indicating the production of work, although the mutant loop is so narrow as to hardly produce any work at all.
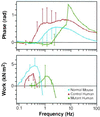
Figure 4
Composite Bode plot showing the response of papillary muscle (three muscles of each type) to oscillations over the frequency range of 0.08–100 Hz. The upper panel shows that the phase becomes negative in human mutant mice (force lags length, indicating work producing) between frequencies of 0.9 and 2.6 Hz. The normal mouse papillary muscles have negative phase between frequencies of 0.3 and 0.5 Hz, with error bars too small to show. Human control mouse papillary muscles have a negative phase below frequencies of the normal mouse. The lower panel shows the mean calculated work per loop for the three muscle types. The peak work/cycle is produced between 0.3 and 0.4 Hz in the normal and control human muscle bundles. Work at this frequency is minimal in the mutant muscle bundles in which the peak work/cycle has been shifted to ≈1.3 Hz.
Figure 5
Comparison of adult transgenic mouse hearts to a normal adult mouse heart. At 1–1.5 years of age, gross cardiac morphology is normal in normal mouse (A) and in hearts from three independent transgenic human control mouse lines (B_–_D); arrows show normal left ventricular cavity. However, the hearts from four independent transgenic mutant human transgenic mouse lines (E_–_H) show a thin apex (arrowhead) and obliteration of the middle of the left ventricular cavity (arrows).
Similar articles
- Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain.
Kazmierczak K, Liang J, Yuan CC, Yadav S, Sitbon YH, Walz K, Ma W, Irving TC, Cheah JX, Gomes AV, Szczesna-Cordary D. Kazmierczak K, et al. FASEB J. 2019 Mar;33(3):3152-3166. doi: 10.1096/fj.201801402R. Epub 2018 Oct 26. FASEB J. 2019. PMID: 30365366 Free PMC article. - Ablation of the N terminus of cardiac essential light chain promotes the super-relaxed state of myosin and counteracts hypercontractility in hypertrophic cardiomyopathy mutant mice.
Sitbon YH, Kazmierczak K, Liang J, Yadav S, Veerasammy M, Kanashiro-Takeuchi RM, Szczesna-Cordary D. Sitbon YH, et al. FEBS J. 2020 Sep;287(18):3989-4004. doi: 10.1111/febs.15243. Epub 2020 Feb 25. FEBS J. 2020. PMID: 32034976 Free PMC article. - Discrete effects of A57G-myosin essential light chain mutation associated with familial hypertrophic cardiomyopathy.
Kazmierczak K, Paulino EC, Huang W, Muthu P, Liang J, Yuan CC, Rojas AI, Hare JM, Szczesna-Cordary D. Kazmierczak K, et al. Am J Physiol Heart Circ Physiol. 2013 Aug 15;305(4):H575-89. doi: 10.1152/ajpheart.00107.2013. Epub 2013 Jun 7. Am J Physiol Heart Circ Physiol. 2013. PMID: 23748425 Free PMC article. - Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers.
Maughan D, Moore J, Vigoreaux J, Barnes B, Mulieri LA. Maughan D, et al. Adv Exp Med Biol. 1998;453:471-80. doi: 10.1007/978-1-4684-6039-1_52. Adv Exp Med Biol. 1998. PMID: 9889859 Review. - Myosin light chain 2 into the mainstream of cardiac development and contractility.
Moss RL, Fitzsimons DP. Moss RL, et al. Circ Res. 2006 Aug 4;99(3):225-7. doi: 10.1161/01.RES.0000236793.88131.dc. Circ Res. 2006. PMID: 16888245 Review. No abstract available.
Cited by
- Overexpression of miniparamyosin causes muscle dysfunction and age-dependant myofibril degeneration in the indirect flight muscles of Drosophila melanogaster.
Arredondo JJ, Mardahl-Dumesnil M, Cripps RM, Cervera M, Bernstein SI. Arredondo JJ, et al. J Muscle Res Cell Motil. 2001;22(3):287-99. doi: 10.1023/a:1012431725009. J Muscle Res Cell Motil. 2001. PMID: 11763201 - Kinetics and energetics of the crossbridge cycle.
Maughan DW. Maughan DW. Heart Fail Rev. 2005 Sep;10(3):175-85. doi: 10.1007/s10741-005-5248-2. Heart Fail Rev. 2005. PMID: 16416041 Review. - Hereditary heart disease: pathophysiology, clinical presentation, and animal models of HCM, RCM, and DCM associated with mutations in cardiac myosin light chains.
Yadav S, Sitbon YH, Kazmierczak K, Szczesna-Cordary D. Yadav S, et al. Pflugers Arch. 2019 May;471(5):683-699. doi: 10.1007/s00424-019-02257-4. Epub 2019 Jan 31. Pflugers Arch. 2019. PMID: 30706179 Free PMC article. Review. - Regulating the contraction of insect flight muscle.
Bullard B, Pastore A. Bullard B, et al. J Muscle Res Cell Motil. 2011 Dec;32(4-5):303-13. doi: 10.1007/s10974-011-9278-1. Epub 2011 Nov 22. J Muscle Res Cell Motil. 2011. PMID: 22105701 Review. - In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament.
Harris SP, Lyons RG, Bezold KL. Harris SP, et al. Circ Res. 2011 Mar 18;108(6):751-64. doi: 10.1161/CIRCRESAHA.110.231670. Circ Res. 2011. PMID: 21415409 Free PMC article. Review.
References
- Huxley A F. Prog Biophys Biophys Chem. 1957;7:225–318. - PubMed
- Huxley H E. Science. 1969;164:1356–1366. - PubMed
- Huxley A F, Simmons R M. Nature (London) 1972;233:533–538. - PubMed
- Swynghedauw B. Physiol Rev. 1986;66:710–771. - PubMed
- McDonald K S, Moss R L. Circ Res. 1995;77:199–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources