Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain - PubMed (original) (raw)
Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain
T Cartmell et al. J Neurosci. 1999.
Abstract
Adenovirus-mediated gene transfer into the brain is associated with significant inflammation and activation of anti-vector and anti-transgene immune responses that curtail the gene delivery of adenoviruses and therapeutic efficacy. Elucidating the molecular mediators of inflammatory and immune responses to adenoviruses injected into the brain should allow us to inhibit their inflammatory actions, thereby reducing vector clearance and enhance adenoviral-mediated gene transfer into the CNS. Cytokines are primary mediators of the immune response and are released during inflammation. Here we report for the first time that injection of replication-deficient adenovirus vectors into the cerebral ventricles of rats causes a rapid increase in body temperature. This fever response precedes any vector-encoded transgene expression and occurs with vectors encoding no transgene, as well as with vectors encoding a therapeutic transgene i.e., HSV1-thymidine kinase. No fever is detected after infection of the striatum, an important brain target in studies on neurodegeneration. After infection of the brain ventricles, CSF levels of immunoreactive tumor necrosis factor (TNF)-alpha and interleukin (IL)-1beta increase significantly (up to 300-fold). In the hypothalamus, the locus of thermoregulation in the brain, only IL-1beta and IL-6 are significantly elevated. A neutralizing TNF-alpha antibody has no effect on adenovirus-induced fever. However, pretreatment with either the IL-1 receptor antagonist or the cyclooxygenase inhibitor flurbiprofen completely abolishes adenovirus-induced fever, suggesting that IL-1 and prostaglandins are direct mediators of this response. These results are the first to demonstrate that IL-1, but not TNF-alpha, is the main mediator of a very early inflammatory response to adenovirus in the brain.
Figures
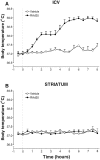
Fig. 1.
A, A replication-deficient recombinant adenovirus expressing β-galactosidase (RAd35) injected intracerebroventricularly (1.3 × 108 pfu in 2 μl) caused a significant increase in core body temperature that was maximal 7 hr after injection (***p < 0.001 vs vehicle; n = 6). _Dotted line_indicates time of injection (0 hr, 10:00 A.M.). B, Striatal injection of the same recombinant adenovirus vector resulted in no significant change in core body temperature (vs vehicle;n = 5) for the duration of the experiment (48 hr).Dotted line indicates time of injection (0 hr).
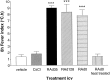
Fig. 2.
Temperature responses to intracerebroventricular (2 μl) injection of 1.3 × 108 pfu/rat of RAd35 (encoding the marker transgene β-galactosidase), RAd0 (containing no transgene), or RAd128 (encoding HSV1-TK) were significantly different from that of vehicle (ANOVA, ***p < 0.001). The concentration of CsCl, as used in the viral purification gradient, was dialyzed against the purification buffers and injected intracerebroventricularly to control for the possible pyrogenic effect of any remaining CsCl in the viral preparation. Neither the vehicle nor CsCl induced fever. Furthermore, heat treatment of RAd0 (30 min at 90°C) completely eliminated the fever response.
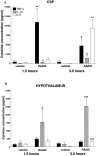
Fig. 3.
A, Intracerebroventricular injection of adenovirus elicited a marked increase in the levels of TNF-α (**p < 0.01) and IL-1β (#p < 0.05) in the CSF 1.5 hr after injection, followed at the 3 hr time point by a reduction in the TNF-α levels (by 61%) and a continued increase in IL-1β levels (#p < 0.05) when compared with vehicle-injected controls. IL-6 increased significantly (**p < 0.01 vs vehicle) 3 hr after adenovirus injection. B, Hypothalamic IL-1β increased significantly at the 1.5 hr time point (#p < 0.05 vs vehicle). Both IL-1β (***p < 0.001) and IL-6 (***p< 0.001) levels were significantly elevated 3 hr after adenovirus injection, compared with vehicle-injected controls.
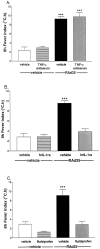
Fig. 4.
A, The temperature response to intracerebroventricular injection of adenovirus (0 hr) after pretreatment with vehicle or TNF-α antiserum was only significantly different (ANOVA; ***p < 0.001) from that of vehicle or TNF-α antiserum injected 24 hr earlier plus vehicle injected at 0 hr. B, The increase in core temperature elicited by adenovirus (i.c.v., 0 hr) was abolished by injection of IL-1ra (200 μg/rat, i.c.v.; 0 and 1 hr). The temperature response to adenovirus was significantly different from that of vehicle, IL-1ra, or adenovirus plus IL-1ra (ANOVA; ***p < 0.001).C, Intracerebroventricular injection of adenovirus (0 hr) elicited a marked and sustained increase in core temperature that was totally abolished by intraperitoneal injection of flurbiprofen (1 mg/kg, −0.5 hr). Data for vehicle, flurbiprofen, or adenovirus plus flurbiprofen were all significantly different from the temperature response to adenovirus (ANOVA; ***p < 0.001).
Similar articles
- Febrile response to tissue inflammation involves both peripheral and brain IL-1 and TNF-alpha in the rat.
Luheshi GN, Stefferl A, Turnbull AV, Dascombe MJ, Brouwer S, Hopkins SJ, Rothwell NJ. Luheshi GN, et al. Am J Physiol. 1997 Mar;272(3 Pt 2):R862-8. doi: 10.1152/ajpregu.1997.272.3.R862. Am J Physiol. 1997. PMID: 9087648 - Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice.
Benihoud K, Esselin S, Descamps D, Jullienne B, Salone B, Bobé P, Bonardelle D, Connault E, Opolon P, Saggio I, Perricaudet M. Benihoud K, et al. Gene Ther. 2007 Mar;14(6):533-44. doi: 10.1038/sj.gt.3302885. Epub 2006 Nov 16. Gene Ther. 2007. PMID: 17109009 - Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression.
Stone D, Xiong W, Williams JC, David A, Lowenstein PR, Castro MG. Stone D, et al. Mol Ther. 2003 Sep;8(3):400-11. doi: 10.1016/s1525-0016(03)00178-3. Mol Ther. 2003. PMID: 12946313 Free PMC article. - Adenovirus in the brain: recent advances of gene therapy for neurodegenerative diseases.
Barkats M, Bilang-Bleuel A, Buc-Caron MH, Castel-Barthe MN, Corti O, Finiels F, Horellou P, Revah F, Sabate O, Mallet J. Barkats M, et al. Prog Neurobiol. 1998 Jul;55(4):333-41. doi: 10.1016/s0301-0082(98)00028-8. Prog Neurobiol. 1998. PMID: 9654383 Review. - Cytokine Responses to Adenovirus and Adenovirus Vectors.
Atasheva S, Shayakhmetov DM. Atasheva S, et al. Viruses. 2022 Apr 24;14(5):888. doi: 10.3390/v14050888. Viruses. 2022. PMID: 35632630 Free PMC article. Review.
Cited by
- LIGHT/TNFSR14 can regulate hepatic lipase expression by hepatocytes independent of T cells and Kupffer cells.
Chellan B, Koroleva EP, Sontag TJ, Tumanov AV, Fu YX, Getz GS, Reardon CA. Chellan B, et al. PLoS One. 2013;8(1):e54719. doi: 10.1371/journal.pone.0054719. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23355893 Free PMC article. - Gene transfer into rat brain using adenoviral vectors.
Puntel M, Kroeger KM, Sanderson NS, Thomas CE, Castro MG, Lowenstein PR. Puntel M, et al. Curr Protoc Neurosci. 2010 Jan;Chapter 4:Unit 4.24. doi: 10.1002/0471142301.ns0424s50. Curr Protoc Neurosci. 2010. PMID: 20066657 Free PMC article. - Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat.
Allan SM, Parker LC, Collins B, Davies R, Luheshi GN, Rothwell NJ. Allan SM, et al. Proc Natl Acad Sci U S A. 2000 May 9;97(10):5580-5. doi: 10.1073/pnas.090464197. Proc Natl Acad Sci U S A. 2000. PMID: 10779559 Free PMC article. - Nonneurotropic adenovirus: a vector for gene transfer to the brain and gene therapy of neurological disorders.
Lowenstein PR, Suwelack D, Hu J, Yuan X, Jimenez-Dalmaroni M, Goverdhana S, Castro MG. Lowenstein PR, et al. Int Rev Neurobiol. 2003;55:3-64. doi: 10.1016/s0074-7742(03)01001-8. Int Rev Neurobiol. 2003. PMID: 12968530 Free PMC article. Review. No abstract available. - Combining cytotoxic and immune-mediated gene therapy to treat brain tumors.
Curtin JF, King GD, Candolfi M, Greeno RB, Kroeger KM, Lowenstein PR, Castro MG. Curtin JF, et al. Curr Top Med Chem. 2005;5(12):1151-70. doi: 10.2174/156802605774370856. Curr Top Med Chem. 2005. PMID: 16248789 Free PMC article. Review.
References
- Adesanya MR, Redman RS, Baum BJ, O’Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. - PubMed
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources