A novel in vivo assay reveals inhibition of ribosomal nuclear export in ran-cycle and nucleoporin mutants - PubMed (original) (raw)
A novel in vivo assay reveals inhibition of ribosomal nuclear export in ran-cycle and nucleoporin mutants
E Hurt et al. J Cell Biol. 1999.
Abstract
To identify components involved in the nuclear export of ribosomes in yeast, we developed an in vivo assay exploiting a green fluorescent protein (GFP)-tagged version of ribosomal protein L25. After its import into the nucleolus, L25-GFP assembles with 60S ribosomal subunits that are subsequently exported into the cytoplasm. In wild-type cells, GFP-labeled ribosomes are only detected by fluorescence in the cytoplasm. However, thermosensitive rna1-1 (Ran-GAP), prp20-1 (Ran-GEF), and nucleoporin nup49 and nsp1 mutants are impaired in ribosomal export as revealed by nuclear accumulation of L25-GFP. Furthermore, overexpression of dominant-negative RanGTP (Gsp1-G21V) and the tRNA exportin Los1p inhibits ribosomal export. The pattern of subnuclear accumulation of L25-GFP observed in different mutants is not identical, suggesting that transport can be blocked at different steps. Thus, nuclear export of ribosomes requires the nuclear/cytoplasmic Ran-cycle and distinct nucleoporins. This assay can be used to identify soluble transport factors required for nuclear exit of ribosomes.
Figures
Figure 1
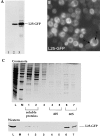
Assembly of L25-GFP into 60S ribosomes. (A) Western blot analysis of whole cell extracts. 1, CHRS 52 strain; 2, CHRS 52 strain transformed with pRS314-L25-GFP (single copy plasmid); 3, CHRS 52 strain transformed with pYEplac112-L25-GFP (high copy plasmid). Equal amounts of cells were loaded on the SDS–polyacrylamide gel that was blotted onto nitrocellulose and probed with anti-GFP antibodies. The position of L25-GFP is shown. (B) Fluorescence microscopy of yeast cells (CHRS 1d) expressing L25-GFP. The vacuole (V) and nuclear compartment (N) is indicated in one of the L25-GFP-expressing cells. (C) Ribosome isolation by sucrose gradient centrifugation. Ribosomal proteins of the 60S and 40S subunits, which were obtained from CHRS 1d cells expressing L25-GFP, were separated by SDS-PAGE and visualized, respectively, by Coomassie-staining (upper panel) and Western blotting using anti-GFP antibodies (lower panel). L, load, i.e., whole cell extract of yeast strain CHRS 1d expressing L25-GFP; M, 10 kD protein ladder standard (the prominent band corresponds to 50 kD); 1–7, fractions from the sucrose gradient. The position of the 60S and 40S fractions, and of the soluble proteins, are indicated.
Figure 2
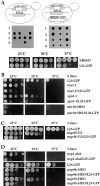
Growth of L25-GFP-expressing strains. (A) L25-GFP complements a rpl25::HIS3 null mutant. (Upper and middle panel) Tetrad analysis of the heterozygous rpl25::HIS3/RPL25 diploid strain reveals a 2:2 segregation, indicating that the RPL25 gene is essential for viability. Viability of the rpl25::HIS3 spores is rescued by cosegregation of the pYEplac195-ADE2-URA3-L25-GFP plasmid with chromosomal rpl25::HIS3. (Lower panel) Growth properties of haploid L25-GFP strain. Precultures were diluted in growth medium and equivalent amounts of cells (diluted in 10−1 steps) were spotted onto YPD plates. hRS453 is a haploid wild-type, L25-GFP a haploid strain disrupted for rlp25:: HIS3 and complemented by YEplac195-ADE2-URA3-L25-GFP. It was grown for 4 d. (B–D) Growth properties of different single and double mutant strains at different temperatures. Precultures were diluted in growth medium and equivalent amounts of cells (diluted in 10−1 steps) were spotted onto YPD plates. Cells were grown at the indicated temperatures and for the indicated days on YPD plates. For description of strains, see Materials and Methods and Table I.
Figure 3
Nuclear accumulation of L25-GFP in rna1-1 mutant. Thermosensitive mutant rna1-1 transformed with YEplac195-ADE2-URA3-L25-GFP (A), and the rpl25::HIS3 disruption mutant rna1-1/L25-GFP (B) were used. Cells were grown at 23°C on selective SDC-ura plates (A) and YPD plates (B) to stationary phase (3–4 d on plate) before they were inoculated in liquid YPD-medium. It was then shifted for the indicated time points to 37°C, before cells were further incubated at room temperature (20°C). After centrifugation, cells were resuspended in water, mounted on a slide and inspected in the fluorescence microscope.
Figure 4
Ribosomal export in prp20-1 and mutants mapping in importin/karyopherin β–like transport factors. Thermosensitive mutants prp20-1, pse1-1/kap123::HIS3, and xpo1-1 were transformed with YEplac195-ADE2-URA3-L25-GFP (pL25-GFP), or the double xpo1-1/L25-GFP strain was constructed (see Table I). Cells were grown at 23°C on selective SDC-ura and YPD plates, respectively, to stationary phase, before they were inoculated in liquid YPD-medium. It was shifted for the indicated time points to 37°C, before cells were further incubated at room temperature (20°C). After centrifugation, cells were resuspended in water, mounted on a slide and inspected in the fluorescence microscope.
Figure 5
Accumulation of L25-GFP inside the nucleus in strains overexpressing Los1p and Gsp1p-G21V. L25-GFP cells carrying the plasmids (A) pEMBLyex4 (GAL), (B) pGAL-GSP1-G21V, (C) pGAL-KAP95, (D) pGAL-YRB4ΔN, (E) pEMBLyex4-ProtA-LOS1 (GAL-LOS1), (F and G) pEMBLyex4-ProtA-LOS1ΔN (GAL-LOS1ΔN) were first grown in raffinose-containing medium before transferring them into galactose-containing medium/ plate (see in Materials and Methods). After growth in galactose for 12 h, the localization of L25-GFP was determined in the fluorescence microscope (A–F). GAL-LOS1ΔN cells were also fixed in 3.7% formaldehyde for 30 min before spheroplasting and indirect immunofluorescence microscopy (G). The L25-GFP signal (fluorescein channel; indicated by arrows), the Nop1p signal (rhodamin channel) and the DNA signal (Hoechst channel) were recorded in a Zeiss Axioskop fluorescence microscope. L25-GFP and Nop1p colocation is indicated by arrows.
Figure 6
Nuclear accumulation of L25-GFP in nup49-313 ts mutant. (A) Time-course of nuclear accumulation of L25-GFP in nup49-313/L25-GFP cells. Cells were first grown at 23°C before shift for 14 h to 33°C. After the restrictive growth condition, cells were incubated at the permissive temperature (20°C) and inspected in the fluorescence microscope after 0, 1, 4, 10, and 24 h. (B) Indirect immunofluorescence microscopy of nup49-313/L25-GFP cells. After fixation of cells in 3.7% formaldehyde, spheroplasted cells were processed for indirect immunofluorescence using anti-Pus1p antibodies. Cells were also stained for DNA with Hoechst 33258. The L25-GFP signal was recorded in the fluorescein channel, the Pus1p immunostaining in the rhodamin channel of a Zeiss Axioskop fluorescence microscope.
Figure 7
Nuclear accumulation of L25-GFP in nucleoporin mutants. Analysis of L25-GFP nuclear accumulation in nucleoporin and ribosome assembly mutants. Haploid double mutants (see Table I) nup49-313/L25-GFP, nsp1-ala6/L25-GFP nup85::HIS3/ L25-GFP, nup84::HIS3/L25-GFP, and nop1-7/L25-GFP were grown as described in Materials and Methods in YPD-medium to OD (600 nm) of 0.2 OD before shift for 14 h to 33°C and reshift for further 4 h to 20°C. Cells were viewed in the fluorescence microscope to see L25-GFP and under Nomarski optics.
Figure 8
NLS-mediated nuclear protein uptake and poly(A)+ RNA export in thermosensitive nup49-313 cells. (A) The thermosensitive nup49-313 mutant was transformed with pGAL-NLS-GFP-lacZ (classical NLS of SV-40 large T antigen) and transformants were selected on SDC (−ura) plates. Transformants were grown in selective synthetic raffinose (−ura) medium at 20°C. One-half of the culture was left at 20°C (right panel), the other half was shifted for 11 h to 37°C (left panel). Cells from both cultures were collected by centrifugation, transfered into in SGal (−ura) medium and incubated for 5 h at 20°C to induce pGAL-NLS-GFP-lacZ expression. Cells were finally inspected in the fluorescence microscope. Note that at both temperatures the nuclear import reporter accumulates in the nucleus. (B) Nuclear export of poly(A)+ RNA in nup49-313/L25-GFP and L25-GFP cells. Cells were grown at the indicated temperatures (14 h at 33°C; 14 h at 33°C, and 4 h at 20°C; 18 h at 20°C) before they were fixed and processed for in situ hybridization using a fluorescently labeled oligo dT-probe.
Figure 9
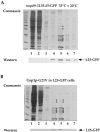
L25-GFP containing ribosomes isolated from nup49-313/L25-GFP and Gsp1p-G21V/L25-GFP cells. Ribosomes were isolated under low salt conditions (100 mM KCl) from (A) nup49-313/L25-GFP cells shifted for 14 h to 33°C and incubated for another 4 h at 20°C and (B) L25-GFP cells expressing Gsp1p-G21V under restrictive conditions (i.e., galactose medium) as described in the legend of Fig. 5 and in Materials and Methods. Whole cell extracts were prepared, loaded on a 10–40% sucrose gradient and centrifuged for 12 h at 150,000 g. The fractions from the sucrose density gradient were TCA-precipitated, resuspended in SDS-sample buffer and analyzed by SDS-PAGE and Coomassie-staining (upper part) or Western blotting using anti-GFP antibodies (lower part). The position of L25-GFP is indicated. The asterisks mark the position of prominent ribosomal proteins. Marker proteins (10-kD ladder with a stronger 50-kD band) are also depicted.
Similar articles
- Factors affecting nuclear export of the 60S ribosomal subunit in vivo.
Stage-Zimmermann T, Schmidt U, Silver PA. Stage-Zimmermann T, et al. Mol Biol Cell. 2000 Nov;11(11):3777-89. doi: 10.1091/mbc.11.11.3777. Mol Biol Cell. 2000. PMID: 11071906 Free PMC article. - Interactions between a nuclear transporter and a subset of nuclear pore complex proteins depend on Ran GTPase.
Seedorf M, Damelin M, Kahana J, Taura T, Silver PA. Seedorf M, et al. Mol Cell Biol. 1999 Feb;19(2):1547-57. doi: 10.1128/MCB.19.2.1547. Mol Cell Biol. 1999. PMID: 9891088 Free PMC article. - Ultrastructural localization of rRNA shows defective nuclear export of preribosomes in mutants of the Nup82p complex.
Gleizes PE, Noaillac-Depeyre J, Léger-Silvestre I, Teulières F, Dauxois JY, Pommet D, Azum-Gelade MC, Gas N. Gleizes PE, et al. J Cell Biol. 2001 Dec 10;155(6):923-36. doi: 10.1083/jcb.200108142. Epub 2001 Dec 10. J Cell Biol. 2001. PMID: 11739405 Free PMC article. - Nuclear export of ribosomal subunits.
Johnson AW, Lund E, Dahlberg J. Johnson AW, et al. Trends Biochem Sci. 2002 Nov;27(11):580-5. doi: 10.1016/s0968-0004(02)02208-9. Trends Biochem Sci. 2002. PMID: 12417134 Review. - Review: transport of tRNA out of the nucleus-direct channeling to the ribosome?
Grosshans H, Simos G, Hurt E. Grosshans H, et al. J Struct Biol. 2000 Apr;129(2-3):288-94. doi: 10.1006/jsbi.2000.4226. J Struct Biol. 2000. PMID: 10806079 Review.
Cited by
- Ran-GTP/-GDP-dependent nuclear accumulation of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 and TGACG-BINDING FACTOR2 controls salicylic acid-induced leaf senescence.
Pham G, Shin DM, Kim Y, Kim SH. Pham G, et al. Plant Physiol. 2022 Jun 27;189(3):1774-1793. doi: 10.1093/plphys/kiac164. Plant Physiol. 2022. PMID: 35417014 Free PMC article. - Protein trans-acting factors involved in ribosome biogenesis in Saccharomyces cerevisiae.
Kressler D, Linder P, de La Cruz J. Kressler D, et al. Mol Cell Biol. 1999 Dec;19(12):7897-912. doi: 10.1128/MCB.19.12.7897. Mol Cell Biol. 1999. PMID: 10567516 Free PMC article. Review. No abstract available. - Dominant mutations in the late 40S biogenesis factor Ltv1 affect cytoplasmic maturation of the small ribosomal subunit in Saccharomyces cerevisiae.
Fassio CA, Schofield BJ, Seiser RM, Johnson AW, Lycan DE. Fassio CA, et al. Genetics. 2010 May;185(1):199-209. doi: 10.1534/genetics.110.115584. Epub 2010 Mar 9. Genetics. 2010. PMID: 20215468 Free PMC article. - Yvh1 is required for a late maturation step in the 60S biogenesis pathway.
Kemmler S, Occhipinti L, Veisu M, Panse VG. Kemmler S, et al. J Cell Biol. 2009 Sep 21;186(6):863-80. doi: 10.1083/jcb.200904111. J Cell Biol. 2009. PMID: 19797079 Free PMC article. - The ribosomal protein Rps15p is required for nuclear exit of the 40S subunit precursors in yeast.
Léger-Silvestre I, Milkereit P, Ferreira-Cerca S, Saveanu C, Rousselle JC, Choesmel V, Guinefoleau C, Gas N, Gleizes PE. Léger-Silvestre I, et al. EMBO J. 2004 Jun 16;23(12):2336-47. doi: 10.1038/sj.emboj.7600252. Epub 2004 May 27. EMBO J. 2004. PMID: 15167894 Free PMC article.
References
- Adam SA, Lobl TJ, Mitchell MA, Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989;337:276–279. - PubMed
- Aebi M, Clark MW, Vijayraghavan U, Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1, which is involved in the control of chromosome condensation. Mol Gen Genet. 1990;224:72–80. - PubMed
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
- Amberg DC, Goldstein AL, Cole CN. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiaerequired for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous