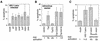Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8 - PubMed (original) (raw)
Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8
S R Bartz et al. J Virol. 1999 Mar.
Abstract
Apoptosis contributes to the loss of CD4 cells during human immunodeficiency virus type 1 (HIV-1) infection. Although the product of the env gene, gp160/gp120, is known to play a role in cell death mediated by HIV-1, the role of other HIV-1 genes in the process is unclear. We found that HIV-1 lacking the env gene (HIVDeltaenv) still induced apoptosis in T-cell lines and primary CD4 T cells. The ability to induce apoptosis was attributable to Tat, a viral regulatory protein. Tat induction of apoptosis was separate from the transactivation function of Tat, required expression of the second exon of Tat, and was associated with the increased expression and activity of caspase-8 (casp-8), a signaling molecule in apoptotic pathways. Moreover, induction of apoptosis could be prevented by treating cells with an inhibitor of casp-8. In addition, we show that HIV-1Deltaenv infection and Tat expression increased the sensitivity of cells to Fas-mediated apoptosis, an apoptotic pathway that signals via casp-8. The up-regulation of casp-8 by HIV-1 Tat expression may contribute to the increased apoptosis and sensitivity to apoptotic signals observed in the cells of HIV-1-infected persons.
Figures
FIG. 1
Method used to analyze HIV-1-induced apoptosis. HIV-1Δ_env_ was pseudotyped with VSV G (A) and used to infect cells at multiplicity of infection that establishes 100% infection (B). (C) Two days postinfection, the cells were analyzed for apoptosis by the TUNEL method using FITC-UTP labeling.
FIG. 2
Genetic analysis of HIV-1- and HIV-1 Tat-induced apoptosis. (A) Mutation of vif, vpr, vpu, rev, and nef does not affect HIV-1-induced apoptosis. Cells were infected with HIV-1Δ_env_/VSV G pseudotypes, which were neither wild type (wt) or mutated in one of the regulatory or accessary genes, and analyzed for apoptosis by TUNEL 2 days postinfection. (B) Full-length HIV-1 Tat but not Tatex1 expressed in the absence of other HIV-1 proteins is able to induce apoptosis. Cells were infected with retroviral vectors that express wild-type or mutant tat genes. Cells expressing Tat were subsequently analyzed for apoptosis by the TUNEL method and for the ability to transactivate the viral LTR. The ability to transactivate the LTR was measured by transfection of an LTR-luciferase construct and is expressed at the bottom as fold activation relative to the value for the empty vector. (C) Full-length Tat but not Tatex1 is able to induce apoptosis when expressed from the HIV-1 provirus. Jurkat cells were infected with an HIV-1Δ_env_/VSV G pseudotype that was either wild type or Δ_rev_ and expressed one- or two-exon Tat. Two days postinfection, the cells were analyzed for apoptosis. The ability of the Δ_rev_ one- and two-exon Tat viruses to transactivate the LTR was assessed by infecting Jurkat cells containing an LTR-luciferase construct. Transactivation ability is expressed at the bottom as fold activation relative to the value for uninfected cells. N.d., not determined. Data in all panels are means of at least three independent experiments.
FIG. 3
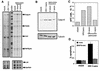
HIV-1 Tat up-regulates casp-8 mRNA and protein activity. (A) The amount of casp-8 RNA is increased in HIV-1-infected cells which express full-length Tat proteins. Jurkat cells were infected with an HIV-1Δ_env_/VSV G pseudotype that was were wild type, Δ_rev_, or Δ_rev_/Tatex1. Two days postinfection, the RNA was isolated and subjected to RPA analysis. Two micrograms of total RNA was analyzed for each sample. Positions of protected RNA fragments for casp-8, FADD, FAF, TNFRp55, L32, and GAPDH are indicated. The gel was analyzed with a PhosphorImager, and the fold increase in specific RNA relative to value for L32 and GAPDH was determined. The L32 and GAPDH blots are shorter exposures of the same gel. The probe lane is undigested probe used for hybridization. Results representative of the analysis of three individual infections are shown. (B) HIV-1-infected cells expressing full-length Tat but not Tatex1 have elevated casp-8 protein levels. Lysates of uninfected and infected Jurkat cells were prepared 2 days postinfection and analyzed for casp-8 protein levels by Western blotting. Each lane was loaded with 1.5 × 105 cells. The fold increase in casp-8 levels was set relative to the tubulin level. The lysates are from the same cells analyzed by RPA in panel A. (C) Cells infected with HIV-1 which express two-exon Tat proteins have elevated levels of casp-8 activity. At 2 days postinfection, 106 infected and uninfected Jurkat cells were lysed and assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The activity is expressed as the amount of AFC released from the AFC-substrate conjugate. The experiment was repeated on at least three separate infections. The results shown are from cells analyzed by RPA and Western blotting. (D) Treatment of HIV-1-infected cells with a casp-8 inhibitor inhibits induction of apoptosis. At 1 day postinfection, mock- or HIV-1Δ_env_-infected cells were treated with IETD-fmk for 16 h and then analyzed for apoptosis.
FIG. 4
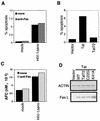
HIV-1 Tat increases sensitivity to Fas-mediated apoptosis. (A) Jurkat cells were infected with HIV-1Δ_env_/VSV G pseudotypes and treated with anti-Fas antibody 2 days postinfection. The amount of apoptosis was detected by TUNEL with FITC-dUTP labeling. (B) HIV-1 expressing full-length but not Tatex1 induces increased sensitivity to Fas-mediated apoptosis. Jurkat cells infected with an HIV-1Δ_env_/VSV G pseudotype that was either wild type, Δ_rev_, or Δ_rev_/Tatex1 were exposed to anti-Fas and analyzed as described above. The data are expressed as percent specific apoptosis, calculated as (amount of apoptosis due to anti-Fas − amount of apoptosis due to no treatment/100 − amount of apoptosis due to no treatment) × 100. (C) Cells infected with HIV-1 which express two-exon Tat proteins have increased casp-8 activity following anti-Fas stimulation. At 2 days postinfection, 106 infected and uninfected Jurkat cells treated with anti-Fas were lysed and assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The activity is expressed as the amount of AFC released from the AFC-substrate conjugate. The analysis of casp-8 activity was performed on lysates of cells analyzed in panel B. Results of a representative experiment of three independent trials are shown. (D) HIV-1 induces apoptosis and increased sensitivity to Fas-mediated apoptosis in primary CD4 T cells. Two days after infection with HIV-1Δ_env_/VSV G, primary CD4 cells were analyzed for expression of HIV-1 p24 antigen and apoptosis. Twenty percent of the cells were infected. Events were acquired until the acquisition of 5,000 p24-positive events. The histograms are from p24-positive and p24-negative cells in the same culture.
FIG. 5
Tat-induced apoptosis is independent of Tat-mediated FasL induction. HIV-1Δ_env_ infection (A) or Tat expression (B) induces apoptosis in Fas-resistant MT4 cells. MT4 cells that were infected with HIV-1Δ_env_/VSV G were analyzed for apoptosis by TUNEL 2 days postinfection. Treatment with anti-Fas had no effect on the level of apoptosis in uninfected or infected cells. Tat- or Tat72-expressing cells were analyzed for apoptosis by TUNEL. Results of a representative experiment of three individual repetitions are shown. (C) HIV-1 induces increased casp-8 activity in Fas-resistant MT4 cells. Two days postinfection, 106 infected or uninfected MT4 cells were assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The results shown are from the analysis of lysates for cells analyzed for apoptosis in panel A. Activity is expressed as the amount of AFC released. (D) Induction of FasL by Tat is separate from the functions of transactivation and induction of apoptosis. RNA from Jurkat cells expressing Tat, Tat72, Tat C22G, or Tat K41A was analyzed for the expression of FasL and actin mRNA by RT-PCR. One microgram of total RNA was used in the RT reaction. WT, wild type.
FIG. 6
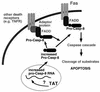
Model of Tat-mediated induction of apoptosis and increased sensitivity to Fas-mediated apoptosis. Tat increases the expression of pro-casp-8 RNA by an undefined mechanism, resulting in increased pro-casp-8 protein, which can result in apoptosis. Sensitivity to Fas-mediated apoptosis is elevated because increased amounts of pro-casp-8 allows for increased recruitment to the occupied receptor.
Similar articles
- The presence of HIV-1 Tat protein second exon delays fas protein-mediated apoptosis in CD4+ T lymphocytes: a potential mechanism for persistent viral production.
López-Huertas MR, Mateos E, Sánchez Del Cojo M, Gómez-Esquer F, Díaz-Gil G, Rodríguez-Mora S, López JA, Calvo E, López-Campos G, Alcamí J, Coiras M. López-Huertas MR, et al. J Biol Chem. 2013 Mar 15;288(11):7626-7644. doi: 10.1074/jbc.M112.408294. Epub 2013 Jan 30. J Biol Chem. 2013. PMID: 23364796 Free PMC article. - HIV-1 Tat protein concomitantly down-regulates apical caspase-10 and up-regulates c-FLIP in lymphoid T cells: a potential molecular mechanism to escape TRAIL cytotoxicity.
Gibellini D, Re MC, Ponti C, Vitone F, Bon I, Fabbri G, Grazia Di Iasio M, Zauli G. Gibellini D, et al. J Cell Physiol. 2005 Jun;203(3):547-56. doi: 10.1002/jcp.20252. J Cell Physiol. 2005. PMID: 15573381 - Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes.
Dabrowska A, Kim N, Aldovini A. Dabrowska A, et al. J Immunol. 2008 Dec 15;181(12):8460-77. doi: 10.4049/jimmunol.181.12.8460. J Immunol. 2008. PMID: 19050264 Free PMC article. - Modulation of apoptosis and viral latency - an axis to be well understood for successful cure of human immunodeficiency virus.
Timilsina U, Gaur R. Timilsina U, et al. J Gen Virol. 2016 Apr;97(4):813-824. doi: 10.1099/jgv.0.000402. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26764023 Review. - Human immunodeficiency virus (HIV)-1 proteins and cytoskeleton: partners in viral life and host cell death.
Matarrese P, Malorni W. Matarrese P, et al. Cell Death Differ. 2005 Aug;12 Suppl 1:932-41. doi: 10.1038/sj.cdd.4401582. Cell Death Differ. 2005. PMID: 15818415 Review.
Cited by
- From Entry to Egress: Strategic Exploitation of the Cellular Processes by HIV-1.
Ramdas P, Sahu AK, Mishra T, Bhardwaj V, Chande A. Ramdas P, et al. Front Microbiol. 2020 Dec 4;11:559792. doi: 10.3389/fmicb.2020.559792. eCollection 2020. Front Microbiol. 2020. PMID: 33343516 Free PMC article. Review. - In-Vitro Subtype-Specific Modulation of HIV-1 Trans-Activator of Transcription (Tat) on RNAi Silencing Suppressor Activity and Cell Death.
Ronsard L, Yousif AS, Ramesh J, Sumi N, Gorman M, Ramachandran VG, Banerjea AC. Ronsard L, et al. Viruses. 2019 Oct 23;11(11):976. doi: 10.3390/v11110976. Viruses. 2019. PMID: 31652847 Free PMC article. - Engineering modular intracellular protein sensor-actuator devices.
Siciliano V, DiAndreth B, Monel B, Beal J, Huh J, Clayton KL, Wroblewska L, McKeon A, Walker BD, Weiss R. Siciliano V, et al. Nat Commun. 2018 May 14;9(1):1881. doi: 10.1038/s41467-018-03984-5. Nat Commun. 2018. PMID: 29760420 Free PMC article. - Development of an Attenuated Tat Protein as a Highly-effective Agent to Specifically Activate HIV-1 Latency.
Geng G, Liu B, Chen C, Wu K, Liu J, Zhang Y, Pan T, Li J, Yin Y, Zhang J, Huang F, Yu F, Chen J, Ma X, Zhou J, Kuang E, Liu C, Cai W, Zhang H. Geng G, et al. Mol Ther. 2016 Sep;24(9):1528-37. doi: 10.1038/mt.2016.117. Epub 2016 Jun 6. Mol Ther. 2016. PMID: 27434587 Free PMC article. - MicroRNA Involvement in Signaling Pathways During Viral Infection.
Barbu MG, Condrat CE, Thompson DC, Bugnar OL, Cretoiu D, Toader OD, Suciu N, Voinea SC. Barbu MG, et al. Front Cell Dev Biol. 2020 Mar 10;8:143. doi: 10.3389/fcell.2020.00143. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32211411 Free PMC article. Review.
References
- Ameisen J C. Programmed cell death and AIDS: from hypothesis to experiment. Immunol Today. 1992;13:388–391. - PubMed
- Ameisen J C, Estaquier J, Idziorek T, De Bels F. Programmed cell death and AIDS pathogenesis: significance and potential mechanisms. Curr Top Microbiol Immunol. 1995;200:195–211. - PubMed
- Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. - PubMed
- Bartz S R, Vodicka M A. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods. 1997;12:337–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous