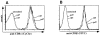T cell affinity maturation by selective expansion during infection - PubMed (original) (raw)
T cell affinity maturation by selective expansion during infection
D H Busch et al. J Exp Med. 1999.
Abstract
T lymphocyte recognition of infected cells is mediated by T cell receptors (TCRs) interacting with their ligands, self-major histocompatibility complex (MHC) molecules complexed with pathogen-derived peptides. Serial TCR interactions with potentially small numbers of MHC/ peptide complexes on infected cells transmit signals that result in T lymphocyte expansion and activation of effector functions. The impact of TCR affinity for MHC/peptide complexes on the rate or extent of in vivo T cell expansion is not known. Here we show that in vivo expansion of complex T cell populations after bacterial infection is accompanied by an increase in their overall affinity for antigen. T cell populations that have undergone additional rounds of in vivo expansion express a narrower range of TCRs, have increased sensitivity for antigen in cytotoxic T lymphocyte assays, and bind MHC/peptide complexes with greater affinity. The selective expansion of higher affinity T cells provides an in vivo mechanism for optimizing the early detection of infected cells.
Figures
Figure 1
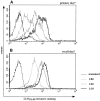
TCR repertoire focusing occurs during T cell expansion. Primary T cells were obtained from the peripheral blood of BALB/c mice 7 d after sublethal infection and stained with LLO91–99 tetramers and a panel of TCR Vβ-specific antibodies (white bars) as described in Materials and Methods. 35 d after primary infection, the same mice were reinfected with a 50-fold higher dose of L. monocytogenes; peripheral blood lymphocytes were isolated and the TCR Vβ repertoire of LLO91–99–specific T cells was determined 5 d after reinfection (black bars). 35 d after reinfection, CD8+ splenocytes from the same mice were isolated and stained with LLO91–99 tetramers and the panel of TCR Vβ-specific antibodies (hatched bars). (A) Representative TCR Vβ profile of an individual mouse during primary and recall infection with L. monocytogenes. (B) The “degree of diversity” decreases from primary to recall LLO91–99–specific T cells, whereas no further changes occur during the transition from recall effector to secondary memory T cells. The degree of diversity was estimated by the ratio of the number of TCR Vβ segments detected in the range of 2.5–10% within an epitope-specific T cell population (gray area in A) divided by the number of TCR Vβ segments detected at >10%. Results from three different mice and standard deviations are shown.
Figure 1
TCR repertoire focusing occurs during T cell expansion. Primary T cells were obtained from the peripheral blood of BALB/c mice 7 d after sublethal infection and stained with LLO91–99 tetramers and a panel of TCR Vβ-specific antibodies (white bars) as described in Materials and Methods. 35 d after primary infection, the same mice were reinfected with a 50-fold higher dose of L. monocytogenes; peripheral blood lymphocytes were isolated and the TCR Vβ repertoire of LLO91–99–specific T cells was determined 5 d after reinfection (black bars). 35 d after reinfection, CD8+ splenocytes from the same mice were isolated and stained with LLO91–99 tetramers and the panel of TCR Vβ-specific antibodies (hatched bars). (A) Representative TCR Vβ profile of an individual mouse during primary and recall infection with L. monocytogenes. (B) The “degree of diversity” decreases from primary to recall LLO91–99–specific T cells, whereas no further changes occur during the transition from recall effector to secondary memory T cells. The degree of diversity was estimated by the ratio of the number of TCR Vβ segments detected in the range of 2.5–10% within an epitope-specific T cell population (gray area in A) divided by the number of TCR Vβ segments detected at >10%. Results from three different mice and standard deviations are shown.
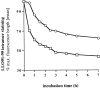
Figure 2
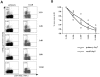
Recall LLO91–99–specific T cells are characterized by higher peptide sensitivity than primary effector CTLs. Short-term T cell lines (A and B) and ex vivo T cells (C and D) were assayed in CTL assays (A and C) or stained for TCR expression (B and D) as described in Materials and Methods. (A) T cell lines were generated by short term in vitro peptide stimulation for 5 d from primary day 7 (□) or reinfected day 5 (○) mice, as described in Materials and Methods, and the percentage of specific lysis in the presence of different concentrations of LLO91–99 peptide was determined by standard 51Cr-release assays using P815 (H2d) target cells. (B) TCR-α/β surface expression of the two T cell lines shown in A. (C) Cr-release assays as in A but with cells isolated directly ex vivo from primary and recall infected mice and enriched for CD8+ T cells. Four mice per group, lines show mean values, symbols show data of individual mice. (D) TCR-α/β surface expression of LLO91–99–specific, primary, or recall T cells isolated directly ex vivo.
Figure 3
High affinity T cell lines bind higher levels of H2-Kd/LLO91–99 tetramers than do low affinity T cells. Short-term T cell lines (see Fig. 2, A and B) were tested for their tetramer-binding capacity by incubating in the presence of different, subsaturating concentrations of H2-Kd/LLO91–99 tetramers for 0.5 h on ice. (A) T cell line from the peak of the primary response to L. monocytogenes; (B) T cell line from the peak of the recall response.
Figure 4
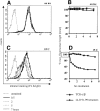
Direct ex vivo tetramer-binding assays. CD8-enriched splenocytes from BALB/c mice at the peak of the primary and recall response to L. monocytogenes were tested in tetramer-binding assays. (A) Dot plots of ex vivo binding for CD8+ primary and recall T cells, showing H2-Kd/LLO91–99 tetramer staining on the y axis and anti-CD62L staining on the x axis. The percentages of tetramer-positive cells with an activated phenotype (CD62Llow) are indicated for the different tetramer concentrations (one representative mouse per group). (B) Changes in the staining intensity of tetramer-positive, CD62Llow T cells (gate R1 in A) at different subsaturating tetramer concentrations in direct ex vivo binding assays. Four mice were used per group; the lines represent the mean values for each group, and symbols show intensity of tetramer positive T cells from individual mice. □, peak primary response; ○, peak recall response.
Figure 5
CD8α/β surface expression of LLO91–99–specific T cell populations. CD8-enriched splenocytes from BALB/c mice at the peak of the primary and recall response to L. monocytogenes were stained for CD8α and CD8β surface expression within the LLO91–99 tetramer–positive T cell population. Cells were first incubated in the presence of anti-CD8 antibodies, followed by staining with NeutrAvidin–PE-conjugated tetramers. Histogram profiles of LLO91–99 tetramer-positive T cells for surface expression of (A) CD8α (Fl-3: CyChr) and (B) CD8β (FL-1: FITC) are shown.
Figure 6
Tetramers dissociate from the cell surface of stained cells. An LLO91–99–specific T cell line was stained on ice with saturating concentrations of H2-Kd/LLO91–99 tetramers, and unbound reagent was washed off. Stained cells were either incubated for 7 h either on ice or at 15°C. Samples were taken at increasing time intervals, fixed, and analyzed for the intensity of tetramer staining. Histograms demonstrate tetramer dissociation (decreased staining intensity) on ice (A) and at 15°C (C). The kinetics of tetramer dissociation and TCR-α/β surface expression on ice (B) or at 15°C (D) are plotted. Tetramer staining is expressed as percentage of maximal staining intensity (time point 0 = 100%); TCR-α/β surface expression was determined by staining of samples on ice after the indicated incubation in the dissociation rate assay and is also expressed as percentage of maximal staining intensity (time point 0 = 100%).
Figure 7
Tetramer dissociation rates of high and low affinity T cell lines. Primary and recall-derived, LLO91–99–specific T cell lines (see Figs. 2 and 3) were tested for tetramer dissociation at 15°C, as described in Fig. 5. □, T cell line from the peak primary response to L. monocytogenes; ○, T cell line from the peak recall response.
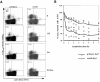
Figure 8
Direct ex vivo tetramer dissociation rates confirm affinity maturation. Tetramer dissociation assays were performed on CD8- enriched splenocytes isolated from BALB/c mice at the peak of the primary or recall response to L. monocytogenes infection. (A) Dot plots of the ex vivo dissociation assays for CD8+ primary and recall T cells, showing H2-Kd/LLO91–99 tetramer staining on the y axis and anti-CD62L staining on the x axis. Percentages of tetramer-positive cells with an activated phenotype (CD62Llow) for the different time points are indicated (one representative mouse per group). (B) The decline in tetramer staining at 15°C of T cells isolated from primary (□) or recall (○) mice is plotted as the percentage of cells that remain in quadrant R1 after the increasing incubation intervals (T0 is assigned a value of 100%). Three mice were tested for each group; the symbols represent the values obtained for CD8+ T cells isolated from individual mice, with lines indicating the mean of three mice during primary and recall infection.
Similar articles
- H2-M3-restricted memory T cells: persistence and activation without expansion.
Kerksiek KM, Ploss A, Leiner I, Busch DH, Pamer EG. Kerksiek KM, et al. J Immunol. 2003 Feb 15;170(4):1862-9. doi: 10.4049/jimmunol.170.4.1862. J Immunol. 2003. PMID: 12574352 - CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo.
Harty JT, Bevan MJ. Harty JT, et al. J Exp Med. 1992 Jun 1;175(6):1531-8. doi: 10.1084/jem.175.6.1531. J Exp Med. 1992. PMID: 1375265 Free PMC article. - Intestinal and splenic T cell responses to enteric Listeria monocytogenes infection: distinct repertoires of responding CD8 T lymphocytes.
Huleatt JW, Pilip I, Kerksiek K, Pamer EG. Huleatt JW, et al. J Immunol. 2001 Mar 15;166(6):4065-73. doi: 10.4049/jimmunol.166.6.4065. J Immunol. 2001. PMID: 11238655 - MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen.
Finelli A, Kerksiek KM, Allen SE, Marshall N, Mercado R, Pilip I, Busch DH, Pamer EG. Finelli A, et al. Immunol Res. 1999;19(2-3):211-23. doi: 10.1007/BF02786489. Immunol Res. 1999. PMID: 10493175 Review. - Acquired immunity to an intracellular pathogen: immunologic recognition of L. monocytogenes-infected cells.
Bouwer HG, Barry RA, Hinrichs DJ. Bouwer HG, et al. Immunol Rev. 1997 Aug;158:137-46. doi: 10.1111/j.1600-065x.1997.tb01000.x. Immunol Rev. 1997. PMID: 9314082 Review.
Cited by
- Biomechanics of T Cell Dysfunctions in Chronic Diseases.
Gunasinghe SD, Peres NG, Goyette J, Gaus K. Gunasinghe SD, et al. Front Immunol. 2021 Feb 25;12:600829. doi: 10.3389/fimmu.2021.600829. eCollection 2021. Front Immunol. 2021. PMID: 33717081 Free PMC article. Review. - Single-cell fate mapping reveals widespread clonal ignorance of low-affinity T cells exposed to systemic infection.
Leube J, Mühlbauer A, Andrä I, Biggel M, Busch DH, Kretschmer L, Buchholz VR. Leube J, et al. Eur J Immunol. 2023 Mar;53(3):e2250009. doi: 10.1002/eji.202250009. Epub 2022 Dec 13. Eur J Immunol. 2023. PMID: 36458456 Free PMC article. - Vaccination reshapes the virus-specific T cell repertoire in unexposed adults.
Pan YG, Aiamkitsumrit B, Bartolo L, Wang Y, Lavery C, Marc A, Holec PV, Rappazzo CG, Eilola T, Gimotty PA, Hensley SE, Antia R, Zarnitsyna VI, Birnbaum ME, Su LF. Pan YG, et al. Immunity. 2021 Jun 8;54(6):1245-1256.e5. doi: 10.1016/j.immuni.2021.04.023. Epub 2021 May 18. Immunity. 2021. PMID: 34004140 Free PMC article. - Synchronizing transcriptional control of T cell metabolism and function.
Man K, Kallies A. Man K, et al. Nat Rev Immunol. 2015 Sep 15;15(9):574-84. doi: 10.1038/nri3874. Epub 2015 Aug 14. Nat Rev Immunol. 2015. PMID: 26272293 Review. - Murine CD8 T-cell functional avidity is stable in vivo but not in vitro: Independence from homologous prime/boost time interval and antigen density.
Gilfillan CB, Wang C, Mohsen MO, Rufer N, Hebeisen M, Allard M, Verdeil G, Irvine DJ, Bachmann MF, Speiser DE. Gilfillan CB, et al. Eur J Immunol. 2020 Apr;50(4):505-514. doi: 10.1002/eji.201948355. Epub 2019 Dec 10. Eur J Immunol. 2020. PMID: 31785153 Free PMC article.
References
- Eisen HN, Siskind CW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. - PubMed
- Jerne NK. A study of avidity based on rabbit skin responses to diphtheria toxin antitoxin mixtures. Acta Path Microbiol Scand. 1951;87(Suppl.):1–183. - PubMed
- Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. - PubMed
- Berek C, Ziegner M. The maturation of the immune response. Immunol Today. 1993;14:400–404. - PubMed
- Nossal GJ. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials