The transcription factor interferon regulatory factor 1 is expressed after cerebral ischemia and contributes to ischemic brain injury - PubMed (original) (raw)
The transcription factor interferon regulatory factor 1 is expressed after cerebral ischemia and contributes to ischemic brain injury
C Iadecola et al. J Exp Med. 1999.
Abstract
The transcription factor interferon regulatory factor 1 (IRF-1) is involved in the molecular mechanisms of inflammation and apoptosis, processes that contribute to ischemic brain injury. In this study, the induction of IRF-1 in response to cerebral ischemia and its role in ischemic brain injury were investigated. IRF-1 gene expression was markedly upregulated within 12 h of occlusion of the middle cerebral artery in C57BL/6 mice. The expression reached a peak 4 d after ischemia (6.0 +/- 1.8-fold; P < 0.001) and was restricted to the ischemic regions of the brain. The volume of ischemic injury was reduced by 23 +/- 3% in IRF-1(+/-) and by 46 +/- 9% in IRF-1(-/-) mice (P < 0.05). The reduction in infarct volume was paralleled by a substantial attenuation in neurological deficits. Thus, IRF-1 is the first nuclear transacting factor demonstrated to contribute directly to cerebral ischemic damage and may be a novel therapeutic target in ischemic stroke.
Figures
Figure 1
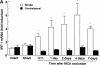
(A) Time course of IRF-1 mRNA expression in mouse cerebral cortex after MCA occlusion. Levels of IRF-1 mRNA were determined by reverse transcription PCR in samples of cerebral cortex ipsilateral (□) or contralateral to the occluded MCA (n = 6–12/time point). mRNA data were normalized to the housekeeping gene HPRT, and are expressed as fold-induction (mean ± SEM) relative to unoperated mice. Since no major differences in IRF-1 mRNA were observed in sham- operated mice killed 12 h and 1, 2, 4, and 7 d after sham operation (n = 1–2/time point), mRNA data from all sham-operated mice were averaged. After MCA occlusion IRF-1 mRNA was markedly upregulated in the ischemic cortex but not contralaterally (*P < 0.05 from contralateral side; Student's t test). (B) A representative Southern blot from the ischemic side of untreated, sham-operated, and MCA-occluded mice (n = 3/group) is shown. Analysis of mRNA for the housekeeping gene HPRT showed no difference among groups in the level of expression (data not shown; see also Fig. 2 A).
Figure 1
(A) Time course of IRF-1 mRNA expression in mouse cerebral cortex after MCA occlusion. Levels of IRF-1 mRNA were determined by reverse transcription PCR in samples of cerebral cortex ipsilateral (□) or contralateral to the occluded MCA (n = 6–12/time point). mRNA data were normalized to the housekeeping gene HPRT, and are expressed as fold-induction (mean ± SEM) relative to unoperated mice. Since no major differences in IRF-1 mRNA were observed in sham- operated mice killed 12 h and 1, 2, 4, and 7 d after sham operation (n = 1–2/time point), mRNA data from all sham-operated mice were averaged. After MCA occlusion IRF-1 mRNA was markedly upregulated in the ischemic cortex but not contralaterally (*P < 0.05 from contralateral side; Student's t test). (B) A representative Southern blot from the ischemic side of untreated, sham-operated, and MCA-occluded mice (n = 3/group) is shown. Analysis of mRNA for the housekeeping gene HPRT showed no difference among groups in the level of expression (data not shown; see also Fig. 2 A).
Figure 2
IRF-1 mRNA expression in cerebral cortex of C57BL/6, IRF-1+/− and IRF-1−/− mice 4 d after MCA occlusion. Levels of IRF-1 mRNA were determined as described in the legend to Fig. 1. (A) Representative Southern blot with three individual mice per group illustrating IRF-1 mRNA expression in wild-type, IRF-1+/−, and IRF-1−/− mice. mRNA for the housekeeping gene HPRT is shown as a control. (B) Group data illustrating IRF-1 mRNA expression in C57BL/6, IRF-1+/−, and IRF-1−/− mice. Means (n = 5/group) are expressed as fold increase relative to the response observed in the nonischemic side (▪) of C57BL/6 mice. IRF-1 mRNA is reduced in IRF-1+/− mice and is absent in IRF-1−/−.
Figure 2
IRF-1 mRNA expression in cerebral cortex of C57BL/6, IRF-1+/− and IRF-1−/− mice 4 d after MCA occlusion. Levels of IRF-1 mRNA were determined as described in the legend to Fig. 1. (A) Representative Southern blot with three individual mice per group illustrating IRF-1 mRNA expression in wild-type, IRF-1+/−, and IRF-1−/− mice. mRNA for the housekeeping gene HPRT is shown as a control. (B) Group data illustrating IRF-1 mRNA expression in C57BL/6, IRF-1+/−, and IRF-1−/− mice. Means (n = 5/group) are expressed as fold increase relative to the response observed in the nonischemic side (▪) of C57BL/6 mice. IRF-1 mRNA is reduced in IRF-1+/− mice and is absent in IRF-1−/−.
Figure 3
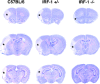
Distribution of the cerebral infarct produced by MCA occlusion in C57BL/6 mice and in IRF-1+/− and IRF-1−/− mice 4 d after MCA occlusion. Thionin-stained representative sections at three different rostrocaudal levels of the mouse brain are presented. The pale areas with asterisks represent the infarcted brain. The infarct size is smaller in IRF-1−/− than in IRF-1+/− or C57BL/6 at all rostrocaudal levels.
Figure 4
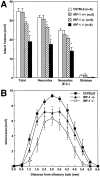
Infarct volume in C57BL/6 and IRF-1 mice 4 d after MCA occlusion. (A) Total infarct volume and infarct volume in neocortex and striatum are presented. Neocortex (E.C.) indicates neocortical infarct volume corrected for swelling (see Materials and Methods for details). Infarct volume (total, neocortex, and striatum) does not differ between C57BL/6 and IRF-1+/+ mice (P > 0.5 analysis of variance and Tukey's test). However, infarct volume is smaller in IRF-1+/− and IRF-1−/− mice (*P < 0.05 from C57BL/6). (B) Rostrocaudal distribution of the area of infarction in the brain of C57BL/6 mice and IRF-1+/− and IRF-1−/− mice 4 d after MCA occlusion. The reduction in infarct area is greatest in IRF-1−/− and is distributed equally at all rostrocaudal levels (*P < 0.05 from C57BL/6; analysis of variance and Tukey's test).
Figure 5
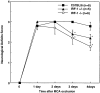
Neurological deficits in C57BL/6, IRF-1+/−, and IRF-1−/− mice after MCA occlusion. Deficits were quantified according to a neurological scale widely used in mice (see Materials and Methods). Deficits were identical in all groups 24 h after MCA occlusion. At 3 and 4 d after MCA occlusion deficits were significantly smaller in IRF-1+/− and IRF-1−/− mice than in C57BL/6 mice (*P < 0.05, Kruskal-Wallis analysis of variance and Tukey's test).
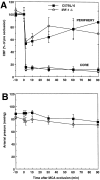
Figure 6
(A) Effect of MCA occlusion on CBF in C57BL/6 mice and in IRF-1−/−. CBF was measured by laser-Doppler flowmetry in the cerebral cortex of anesthetized artificially ventilated mice (n = 5–8/group) with monitoring of arterial pressure and controlled arterial blood gases (see Materials and Methods for details). CBF recordings were made in the center of the ischemic territory, both where the CBF reduction was greatest (core) and toward the edge of the ischemic area (periphery). MCA occlusion produces reduction in CBF that are comparable in C57BL/6 and in IRF-1−/− mice in both the ischemic core and the periphery (P > 0.05). (B) Mean arterial pressure in wild-type and IRF-1−/− mice before and after MCA occlusion. No significant differences in arterial pressure were observed (P > 0.05).
Similar articles
- Interferon regulatory factor-1 immunoreactivity in neurons and inflammatory cells following ischemic stroke in rodents and humans.
Alexander M, Forster C, Sugimoto K, Clark HB, Vogel S, Ross ME, Iadecola C. Alexander M, et al. Acta Neuropathol. 2003 May;105(5):420-4. doi: 10.1007/s00401-002-0658-x. Epub 2002 Dec 18. Acta Neuropathol. 2003. PMID: 12677441 - Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke.
Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Caso JR, et al. Circulation. 2007 Mar 27;115(12):1599-608. doi: 10.1161/CIRCULATIONAHA.106.603431. Epub 2007 Mar 19. Circulation. 2007. PMID: 17372179 - Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene.
Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Iadecola C, et al. J Neurosci. 1997 Dec 1;17(23):9157-64. doi: 10.1523/JNEUROSCI.17-23-09157.1997. J Neurosci. 1997. PMID: 9364062 Free PMC article. - Pivotal role of cerebral interleukin-23 during immunologic injury in delayed cerebral ischemia in mice.
Zheng Y, Zhong D, Chen H, Ma S, Sun Y, Wang M, Liu Q, Li G. Zheng Y, et al. Neuroscience. 2015 Apr 2;290:321-31. doi: 10.1016/j.neuroscience.2015.01.041. Epub 2015 Jan 28. Neuroscience. 2015. PMID: 25637493 - Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development.
Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kündig TM, Amakawa R, Kishihara K, Wakeham A, et al. Matsuyama T, et al. Cell. 1993 Oct 8;75(1):83-97. Cell. 1993. PMID: 8402903
Cited by
- Chronic hypoxia-induced alterations of key enzymes of glucose oxidative metabolism in developing mouse liver are mTOR dependent.
Dukhande VV, Sharma GC, Lai JC, Farahani R. Dukhande VV, et al. Mol Cell Biochem. 2011 Nov;357(1-2):189-97. doi: 10.1007/s11010-011-0889-z. Epub 2011 May 28. Mol Cell Biochem. 2011. PMID: 21625955 - Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage.
Yi JH, Park SW, Kapadia R, Vemuganti R. Yi JH, et al. Neurochem Int. 2007 Jun;50(7-8):1014-27. doi: 10.1016/j.neuint.2007.04.019. Epub 2007 May 3. Neurochem Int. 2007. PMID: 17532542 Free PMC article. Review. - Post-stroke inflammatory response: effects of stroke evolution and outcome.
Tan KT, Lip GY, Blann AD. Tan KT, et al. Curr Atheroscler Rep. 2003 Jul;5(4):245-51. doi: 10.1007/s11883-003-0046-6. Curr Atheroscler Rep. 2003. PMID: 12793964 Review. - Role of histamine and its receptors in cerebral ischemia.
Hu WW, Chen Z. Hu WW, et al. ACS Chem Neurosci. 2012 Apr 18;3(4):238-47. doi: 10.1021/cn200126p. Epub 2012 Feb 10. ACS Chem Neurosci. 2012. PMID: 22860191 Free PMC article. Review. - The science of stroke: mechanisms in search of treatments.
Moskowitz MA, Lo EH, Iadecola C. Moskowitz MA, et al. Neuron. 2010 Jul 29;67(2):181-98. doi: 10.1016/j.neuron.2010.07.002. Neuron. 2010. PMID: 20670828 Free PMC article. Review.
References
- Feuerstein, G.Z., X. Wang and F.C. Barone. 1998. Inflammatory mediators and brain injury: The role of cytokines and chemokines in stroke and CNS diseases. In Cerebrovascular Diseases. M.D. Ginsberg and J. Bogousslavsky, editors. Blackwell Science, Cambridge, MA. 507–531.
- Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocyte/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–1379. - PubMed
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–134. - PubMed
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. - PubMed
- Wang X, Yue TL, Young PR, Barone FC, Feuerstein GZ. Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. J Cereb Blood Flow Metabol. 1995;15:166–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034179/NS/NINDS NIH HHS/United States
- R01 NS035806/NS/NINDS NIH HHS/United States
- NS35806/NS/NINDS NIH HHS/United States
- R37 NS034179/NS/NINDS NIH HHS/United States
- NS34179/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases