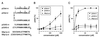Receptor-induced polymerization of coatomer - PubMed (original) (raw)
Receptor-induced polymerization of coatomer
C Reinhard et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Coatomer, the coat protein complex of COPI vesicles, is involved in the budding of these vesicles, but the underlying mechanism is unknown. Toward a better understanding of this process, the interaction between coatomer and the cytoplasmic domain of a major transmembrane protein of COPI vesicles, p23, was studied. Interaction of coatomer with this peptide domain results in a conformational change and polymerization of the complex in vitro. This changed conformation also is observed in vivo, i.e., on the surface of authentic, isolated COPI vesicles. An average of four peptides was found associated with one coatomer complex after polymerization. Based on these results, we propose a mechanism by which the induced conformational change of coatomer results in its polymerization, and thus drives formation of the bud on the Golgi membrane during biogenesis of a COPI vesicle.
Figures
Figure 1
Precipitation of coatomer with synthetic peptides corresponding to the COOH termini of p23wt, p23AS and Wbp1p. (A) Peptides used in this study. p23wt represents the cytoplasmic domain of p23, which binds coatomer depending on its dilysine and diphenylalanine motifs, whereas p23AS lacks these residues and therefore does not bind coatomer. Wbp1p represents the cytoplasmic domain of a subunit of the yeast _N-_oligosaccharyl transferase complex bearing a characteristic KKXX ER-retrieval motif known to bind coatomer. (B and C) Precipitation of coatomer (0.09 μM) with monomeric (B) and dimeric (C) peptides. Only p23wt (■) precipitates the complex, whereas mutated p23 peptide (p23AS, ●) and Wbp1p (▴) have no effect. The error bars indicate standard errors (n = 3).
Figure 2
Limited proteolysis of coatomer. Proteolysis of coatomer (0.09 μM) with thermolysin (0.008 μM) in the presence of dimerized peptides (50 μM) p23wt-d (A), p23AS-d (B), and Wbp1p-d (C). Western blot analysis of proteolytic fragments of the γ-COP subunit reveals a better susceptibility of this subunit to the protease in the presence of p23wt-d (A, lanes 2 and 3) as compared with p23AS-d (B, lanes 2 and 3 h) and Wbp1p-d (C, lanes 2 and 3 h).
Figure 3
Limited proteolysis of COPI vesicles. (A) Suspension of purified COPI vesicles containing ≈0.009 μM coatomer treated with thermolysin (0.009 μM) in the presence of p23AS-d (50 μM) as a substrate for the protease. Western blot analysis of proteolytic fragments of γ-COP reveals a prominent fragment of about 59 kDa (lane 1 h) that is highly susceptible to the protease (lane 2 h). (B) Coatomer (0.009 μM) incubated with p23wt-d (50 μM) and treated with thermolysin under the same conditions as used for the COPI vesicles yields a result comparable to that of COPI vesicles (lanes 1 and 2 h). In contrast, after partial digestion in the presence of p23AS-d (50 μM) of coatomer, the 59-kDa fragment is more stable (C, lanes 1 and 2 h).
Figure 4
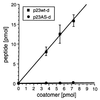
Stoichiometry between coatomer and p23wt-d.125I-labeled p23wt-d (■) or p23AS-d (●) were incubated with increasing concentrations of coatomer. After centrifugation, the amounts of peptide in the precipitates were determined by counting the 125I radioactivity. The error bars indicate standard errors (n = 4).
Figure 5
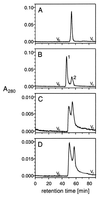
Size exclusion chromatography of p23-tail peptides. (A) Monomeric p23wt (p23wt-m: 1 mg/ml; sample corresponds to 20 μl). (B) Dimeric p23wt (p23wt-d: 1 mg/ml; sample corresponds to 20 μl). Peak 1 represents the dimerized form of p23wt-d, and peak 2 represents the monomeric form of p23wt-d. (C) Rechromatography of peak 1 of B (40 μl). The appearance of both peaks 1 and 2 demonstrates an equilibrium between two states of p23wt-d. (D) Dimeric p23wt (p23wt-d: 0.1 mg/ml; sample corresponds to 40 μl). The concentration dependence of the equilibrium of peak 1 and 2 indicates a bimolecular event.
Figure 6
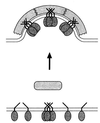
Hypothetical model for bud formation. According to this model, interaction of coatomer with a tetramer of p23 induces a conformational change of the complex. This leads to its polymerization on the surface of a membrane, resulting in the formation of a coated bud. (Note that for simplicity, a role of ARF 1 is not included in this diagram.)
Similar articles
- Structure of the cytoplasmic domain of p23 in solution: implications for the formation of COPI vesicles.
Weidler M, Reinhard C, Friedrich G, Wieland FT, Rösch P. Weidler M, et al. Biochem Biophys Res Commun. 2000 May 10;271(2):401-8. doi: 10.1006/bbrc.2000.2511. Biochem Biophys Res Commun. 2000. PMID: 10799309 - A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding.
Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. Sohn K, et al. J Cell Biol. 1996 Dec;135(5):1239-48. doi: 10.1083/jcb.135.5.1239. J Cell Biol. 1996. PMID: 8947548 Free PMC article. - A single binding site for dilysine retrieval motifs and p23 within the gamma subunit of coatomer.
Harter C, Wieland FT. Harter C, et al. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):11649-54. doi: 10.1073/pnas.95.20.11649. Proc Natl Acad Sci U S A. 1998. PMID: 9751720 Free PMC article. - Peroxisome biogenesis: where Arf and coatomer might be involved.
Lay D, Gorgas K, Just WW. Lay D, et al. Biochim Biophys Acta. 2006 Dec;1763(12):1678-87. doi: 10.1016/j.bbamcr.2006.08.036. Epub 2006 Aug 30. Biochim Biophys Acta. 2006. PMID: 17023067 Review. - Regulation of membrane traffic in animal cells by COPI.
Lowe M, Kreis TE. Lowe M, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):53-66. doi: 10.1016/s0167-4889(98)00046-9. Biochim Biophys Acta. 1998. PMID: 9714733 Review.
Cited by
- p24 family proteins: key players in the regulation of trafficking along the secretory pathway.
Pastor-Cantizano N, Montesinos JC, Bernat-Silvestre C, Marcote MJ, Aniento F. Pastor-Cantizano N, et al. Protoplasma. 2016 Jul;253(4):967-85. doi: 10.1007/s00709-015-0858-6. Epub 2015 Jul 30. Protoplasma. 2016. PMID: 26224213 Review. - Oligomerization and dissociation of AP-1 adaptors are regulated by cargo signals and by ArfGAP1-induced GTP hydrolysis.
Meyer DM, Crottet P, Maco B, Degtyar E, Cassel D, Spiess M. Meyer DM, et al. Mol Biol Cell. 2005 Oct;16(10):4745-54. doi: 10.1091/mbc.e05-06-0568. Epub 2005 Aug 10. Mol Biol Cell. 2005. PMID: 16093346 Free PMC article. - The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast.
Robinson M, Poon PP, Schindler C, Murray LE, Kama R, Gabriely G, Singer RA, Spang A, Johnston GC, Gerst JE. Robinson M, et al. Mol Biol Cell. 2006 Apr;17(4):1845-58. doi: 10.1091/mbc.e05-09-0832. Epub 2006 Feb 1. Mol Biol Cell. 2006. PMID: 16452633 Free PMC article. - Essential lysine residues within transmembrane helix 1 of diphtheria toxin facilitate COPI binding and catalytic domain entry.
Trujillo C, Taylor-Parker J, Harrison R, Murphy JR. Trujillo C, et al. Mol Microbiol. 2010 May;76(4):1010-9. doi: 10.1111/j.1365-2958.2010.07159.x. Epub 2010 Apr 14. Mol Microbiol. 2010. PMID: 20398220 Free PMC article. - COPI budding within the Golgi stack.
Popoff V, Adolf F, Brügger B, Wieland F. Popoff V, et al. Cold Spring Harb Perspect Biol. 2011 Nov 1;3(11):a005231. doi: 10.1101/cshperspect.a005231. Cold Spring Harb Perspect Biol. 2011. PMID: 21844168 Free PMC article. Review.
References
- Rothman J E. Nature (London) 1994;372:55–63. - PubMed
- Rothman J E, Wieland F T. Science. 1996;272:227–234. - PubMed
- Schekman R, Orci L. Science. 1996;271:1526–1533. - PubMed
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn R A, Rothman J E. Cell. 1991;67:239–253. - PubMed
- Taylor T C, Kahn R A, Melancon P. Cell. 1992;70:69–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources