Confocal fluorescence coincidence analysis: an approach to ultra high-throughput screening - PubMed (original) (raw)
Confocal fluorescence coincidence analysis: an approach to ultra high-throughput screening
T Winkler et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Fluorescence-based assay technologies play an increasing role in high-throughput screening. They can be classified into different categories: fluorescence polarization, time-resolved fluorescence, fluorescence resonance energy transfer, and fluorescence correlation spectroscopy. In this work we present an alternative analytical technique for high-throughput screening, which we call confocal fluorescence coincidence analysis. Confocal fluorescence coincidence analysis extracts fluorescence fluctuations that occur coincidently in two different spectral ranges from a tiny observation volume of below 1 fl. This procedure makes it possible to monitor whether an association between molecular fragments that are labeled with different fluorophores is established or broken. Therefore, it provides access to the characterization of a variety of cleavage and ligation reactions in biochemistry. Confocal fluorescence coincidence analysis is a very sensitive and ultrafast technique with readout times of 100 ms and below. This feature is demonstrated by means of a homogeneous assay for restriction endonuclease EcoRI. The presented achievements break ground for throughput rates as high as 10(6) samples per day with using only small amounts of sample substance and therefore constitute a solid base for screening applications in drug discovery and evolutionary biotechnology.
Figures
Figure 1
CFCA setup. Fluorescence excitation was achieved by epi-illumination of a water-immersion objective (UPLAPO 60×/1.2 W; Olympus, Tokyo) with radiation of wavelengths 476/483 nm and 647 nm from a krypton ion laser (INNOVA 90-K; Coherent, Palo Alto, CA) operating in multiline mode. The dichroic beamsplitter A (AHF Analysentechnik, Tübingen, Germany) reflected at <502 nm and 585–655 nm and transmitted at 502–585 and >655 nm. Additional laser lines at 531 nm and 568 nm were blocked with a custom-made excitation/notch filter (>OD 5; AHF Analysentechnik). Appropriate relative laser excitation power for both wavelengths was obtained by the combination of an absorption glass filter (BG 40; Andover Corporation, Salem, NH) and an attenuation filter (OD 0.6; Spindler & Hoyer, Göttingen, Germany). The sample holder was connected to a high-speed two-dimension piezo actor (Piezosystem Jena), which in turn was mounted onto a high-precision x–y mechanical scan table (Märzhäuser). Fluorescence photons were separated at dichroic beamsplitter B (585DCLP02; Omega Optical, Brattleboro, VT) and, after filtering in the red (667EFLP; Omega) and the green channel (535RDF45; Omega), were imaged to two avalanche photo diodes (APD) (SPCM-AQ 131-FS; EG & G, Quebec). Digital pulses of APDs were recorded either by a dual input multiscaler PC card (MCD-2; FAST ComTec, München, Germany) for analysis of time traces at high temporal resolution, or by an on-board processor PC card (Adwin-9LD; Jäger Meβtechnik, Lorsch, Germany), which was programmed to perform on-line data processing. Alternatively, for comparison with dual-color FCS, the APD pulses were crosscorrelated by a PC correlator card (ALV-5000 multiple-τ; ALV, Langen, Germany).
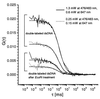
Figure 2
Dual-color FCS curves measured with the described confocal setup (correlation times 60 s) and results of the fitting procedure (see Eq. 1). The parameters obtained from the fitting routine are given in Table 1. The two pairs of curves correspond to two different laser excitation powers (measured at the objective aperture). Each pair describes the dual-color FCS curve measured from a sample of 10 nM double-labeled dsDNA molecules (upper curves) and the curve measured from an equivalent sample after_Eco_RI endonuclease treatment (lower curves), respectively. [Smaller G(0) values at lower excitation power resulted from insufficient saturation of the sample solution.] The experimental cross-correlation curves and the results of the fitting procedure indicated that the optical setup used was well suited to perform a dual-color confocal fluorescence coincidence analysis, as it is presented in this work.
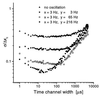
Figure 3
Relative standard deviation σ/Δxc of Gaussian curve fits applied to distributions of K as a function of the time channel width at different frequencies of sample oscillations: K was evaluated from multiscaler time traces with analysis times per sample of 500 ms. At every frequency, there was a range of minimum σ/Δxc in the central part of the covered time channel width. At time channel widths that were in the order of magnitude of the triplet state lifetime (<5 μs) or that were larger than the average residence time τres (which was a function of oscillation frequency), the relative standard deviation increased significantly. The effect of increased frequency was even more pronounced. As the frequency increased, σ/Δxc diminished drastically. Simultaneously, the plateau of time channel width at minimum relative standard deviation narrowed down to the range of 10–30 μs at 216 Hz.
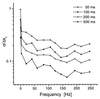
Figure 4
Relative standard deviation σ/Δxc of Gaussian curve fits applied to distributions of K vs. sample oscillation frequency at analysis times ranging from 50 to 500 ms. The abscissa indicates the frequency in y-direction, which was superimposed by a constant frequency of 3 Hz in x-direction (except for the oscillation-free case). Coincidence analysis was performed by using the on-board processor PC card with a time channel width of 12.5 μs. The curves showed similar courses with a large reduction in relative standard deviation when the oscillation of the sample was started. Toward higher frequencies, a further moderate decrease could be noticed. At shortened analysis times the curves were shifted in parallel toward increasing σ/Δxc.
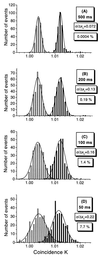
Figure 5
Histogram plots of 300 measurements of the coincidence quantity K at sample oscillation frequencies 216 Hz (y) and 3 Hz (x) (superimposed) in samples containing pure substrate (10 nM double-labeled dsDNA, black bars) and cleaved products (endonuclease-treated 10 nM double-labeled dsDNA, gray bars), respectively. The slices (A–D) are related to different analysis times. The distributions were fitted with Gaussian functions. As a measure of detection error, the relative standard deviation σ/Δxc and the overlap in percentage between corresponding fit functions were calculated.
Similar articles
- A fluorometric assay for DNA cleavage reactions characterized with BamHI restriction endonuclease.
Lee SP, Porter D, Chirikjian JG, Knutson JR, Han MK. Lee SP, et al. Anal Biochem. 1994 Aug 1;220(2):377-83. doi: 10.1006/abio.1994.1353. Anal Biochem. 1994. PMID: 7978282 - Real-time enzyme kinetics monitored by dual-color fluorescence cross-correlation spectroscopy.
Kettling U, Koltermann A, Schwille P, Eigen M. Kettling U, et al. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1416-20. doi: 10.1073/pnas.95.4.1416. Proc Natl Acad Sci U S A. 1998. PMID: 9465029 Free PMC article. - Rapid assay processing by integration of dual-color fluorescence cross-correlation spectroscopy: high throughput screening for enzyme activity.
Koltermann A, Kettling U, Bieschke J, Winkler T, Eigen M. Koltermann A, et al. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1421-6. doi: 10.1073/pnas.95.4.1421. Proc Natl Acad Sci U S A. 1998. PMID: 9465030 Free PMC article. - Mechanism of specific site location and DNA cleavage by EcoR I endonuclease.
Terry BJ, Jack WE, Modrich P. Terry BJ, et al. Gene Amplif Anal. 1987;5:103-18. Gene Amplif Anal. 1987. PMID: 3333364 Review. No abstract available. - [Interactions between Eco RI restriction endonuclease and DNA].
Koziołkiewicz M. Koziołkiewicz M. Postepy Biochem. 1991;37(1):23-32. Postepy Biochem. 1991. PMID: 1896406 Review. Polish. No abstract available.
Cited by
- Semiconductor Nanocrystals for Biological Imaging and Fluorescence Spectroscopy.
Fujii F. Fujii F. Adv Exp Med Biol. 2021;1310:449-473. doi: 10.1007/978-981-33-6064-8_16. Adv Exp Med Biol. 2021. PMID: 33834445 - Circumvention of fluorophore photobleaching in fluorescence fluctuation experiments: a beam scanning approach.
Satsoura D, Leber B, Andrews DW, Fradin C. Satsoura D, et al. Chemphyschem. 2007 Apr 23;8(6):834-48. doi: 10.1002/cphc.200600589. Chemphyschem. 2007. PMID: 17394281 Free PMC article. - Quantitation of ten 30S ribosomal assembly intermediates using fluorescence triple correlation spectroscopy.
Ridgeway WK, Millar DP, Williamson JR. Ridgeway WK, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13614-9. doi: 10.1073/pnas.1204620109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869699 Free PMC article. - The standard deviation in fluorescence correlation spectroscopy.
Wohland T, Rigler R, Vogel H. Wohland T, et al. Biophys J. 2001 Jun;80(6):2987-99. doi: 10.1016/S0006-3495(01)76264-9. Biophys J. 2001. PMID: 11371471 Free PMC article. - Total internal reflection with fluorescence correlation spectroscopy: combined surface reaction and solution diffusion.
Starr TE, Thompson NL. Starr TE, et al. Biophys J. 2001 Mar;80(3):1575-84. doi: 10.1016/S0006-3495(01)76130-9. Biophys J. 2001. PMID: 11222318 Free PMC article.
References
- Magde D, Elson E L, Webb W W. Biopolymers. 1974;13:29–61. - PubMed
- Rigler R, Mets U, Widengren J, Kask P. Eur Biophys J. 1993;22:169–175.
- Auer M, Moore K J, Meyer-Almes F-J, Guenther R, Pope A J, Stoeckli K A. Drug Discovery Today. 1998;3:457–465.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous