Synergy between an antiangiogenic integrin alphav antagonist and an antibody-cytokine fusion protein eradicates spontaneous tumor metastases - PubMed (original) (raw)
Synergy between an antiangiogenic integrin alphav antagonist and an antibody-cytokine fusion protein eradicates spontaneous tumor metastases
H N Lode et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The suppression and eradication of primary tumors and distant metastases is a major goal of alternative treatment strategies for cancer, such as inhibition of angiogenesis and targeted immunotherapy. We report here a synergy between two novel monotherapies directed against vascular and tumor compartments, respectively, a tumor vasculature-specific antiangiogenic integrin alphav antagonist and tumor-specific antibody-interleukin 2 (IL-2) fusion proteins. Simultaneous and sequential combination of these monotherapies effectively eradicated spontaneous liver metastases in a poorly immunogenic syngeneic model of neuroblastoma. This was in contrast to controls subjected to monotherapies with either an antiangiogenic integrin alphav antagonist or antibody-IL-2 fusion proteins, which were only partially effective at the dose levels applied. Furthermore, simultaneous treatments with the integrin alphav antagonist and tumor-specific antibody-IL-2 fusion proteins induced dramatic primary tumor regressions in three syngeneic murine tumor models, i.e., melanoma, colon carcinoma, and neuroblastoma. However, each agent used as monotherapy induced only a delay in tumor growth. A mechanism for this synergism was suggested because the antitumor response was accompanied by a simultaneous 50% reduction in tumor vessel density and a 5-fold increase in inflammatory cells in the tumor microenvironment. Subsequently, tumor necrosis was demonstrated only in animals receiving the combination therapy, but not when each agent was applied as monotherapy. The results suggest that these synergistic treatment modalities may provide a novel and effective tool for future therapies of metastatic cancer.
Figures
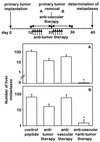
Figure 1
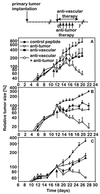
Effect of a combined therapy with antiangiogenic αv integrin antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion proteins on primary tumors. Primary tumors were induced by s.c. injection (2 × 106) of each NXS2 neuroblastoma (A), CT26-KSA colon carcinoma (B), and B78-D14 melanoma cells (C). (Top) Treatment of established tumors (110–130 mm3) by daily i.v. injections of tumor-specific antibody–IL-2 fusion proteins huKS1/4-IL-2 (10 μg, colon carcinoma) and ch14.18-IL-2 (5 μg, neuroblastoma; 10 μg, melanoma) (5×) and continuous s.c. infusion of the vasculature-specific integrin αv antagonist or the control peptide with an osmotic pump for 7 days at 17.5 μg/h. The time of treatment initiation is indicated by a solid arrow. The size of the primary tumors of mice in each experimental group (n = 6) was determined by microcaliper measurements (width × length × width/2) (mean ± SE). The regression in primary tumor size of mice receiving the combination treatment compared with the size of established tumors at the time of treatment initiation was statistically significant in the three different syngeneic tumor models (P < 0.001, Wilcoxon Rank–Sum Test) in contrast to all controls (_P_ > 0.05).
Figure 2
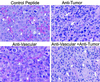
Histology after combined antiangiogenic and tumor-specific immunotherapy of established primary neuroblastoma tumors, surgically removed 20 days after tumor cell inoculation. Formalin-fixed primary tumors were subjected to paraffin embedding and subsequent hematoxylin/eosin staining. Arrowheads delineate blood vessels. Necrotic areas and leukocyte infiltrates are indicated by open stars and arrows, respectively. Representative areas were photographed at ×630.
Figure 3
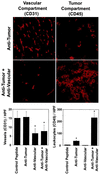
Effect of combined antivascular and antitumor therapies on vascularization and antitumor immune response. Mice (n = 6) with established primary neuroblastoma tumors received the combination treatment, as described in Fig. 1, including controls that received each therapy alone. At the end of the treatment, s.c. tumors were removed surgically. Frozen sections of each tumor were analyzed by immunohistochemistry by using antibodies specific for blood vessel endothelial cells (CD31) and for leukocyte infiltration (CD45), respectively. The latter is a well established marker for the tumor compartment-specific immune response induced by the ch14.18-IL-2 fusion protein (–12). (Left) Blood vessel density of primary tumors after vascular and tumor compartment treatment with either the integrin αv antagonist, ch14.18-IL-2 fusion protein, or a combination thereof (∗,P < 0.001, Student’s t test). (Right) Leukocyte infiltration of primary tumors after vascular and tumor compartment treatments, respectively (∗,P < 0.001, Student’s t test).
Figure 4
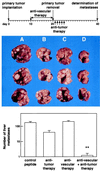
Effect of a sequential combination of antiangiogenic integrin αv antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion protein on spontaneous hepatic neuroblastoma metastases. The antivascular treatment was initiated in mice with established primary tumors, as indicated in Fig. 1, for a total of 10 days (Top). After surgical removal of the primary tumors, mice received the tumor compartment-specific immunotherapy by daily i.v. injections of 5 μg ch14.18-IL-2 fusion protein (5×). Three representative specimens of each treatment group are depicted. (A) Peptide control. (B) Antitumor therapy (ch14.18-IL-2). (C) Antivascular therapy (integrin αv antagonist). (D) Combination of B and C. The number of spontaneous liver metastases was determined by macroscopic counts of liver foci (n = 8) (Bottom) (∗, P < 0.01, Wilcoxon Rank–Sum Test).
Figure 5
Effect of the simultaneous combination of antiangiogenic integrin αv antagonist and antitumor compartment-specific immunotherapy with antibody–IL-2 fusion protein on spontaneous hepatic neuroblastoma metastases. Spontaneous metastases were induced after induction of primary tumors with 2 × 106 NXS2 neuroblastoma cells s.c. (Top). Treatment with integrin αv antagonist (17.5 μg/h) and tumor-specific ch14.18-IL-2 fusion protein (5 × 5 μg) was initiated before (A) or after (B) removal of the primary tumor. Spontaneous liver metastases were determined by macroscopic counts of liver foci (n = 8) (∗,P < 0.01, Wilcoxon Rank–Sum Test).
Similar articles
- Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy.
Xiang R, Lode HN, Dolman CS, Dreier T, Varki NM, Qian X, Lo KM, Lan Y, Super M, Gillies SD, Reisfeld RA. Xiang R, et al. Cancer Res. 1997 Nov 1;57(21):4948-55. Cancer Res. 1997. PMID: 9354462 - Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow.
Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Lode HN, et al. J Natl Cancer Inst. 1997 Nov 5;89(21):1586-94. doi: 10.1093/jnci/89.21.1586. J Natl Cancer Inst. 1997. PMID: 9362156 - Eradication of human hepatic and pulmonary melanoma metastases in SCID mice by antibody-interleukin 2 fusion proteins.
Becker JC, Pancook JD, Gillies SD, Mendelsohn J, Reisfeld RA. Becker JC, et al. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2702-7. doi: 10.1073/pnas.93.7.2702. Proc Natl Acad Sci U S A. 1996. PMID: 8610104 Free PMC article. - Immunocytokines: a new approach to immunotherapy of melanoma.
Reisfeld RA, Becker JC, Gillies SD. Reisfeld RA, et al. Melanoma Res. 1997 Aug;7 Suppl 2:S99-106. Melanoma Res. 1997. PMID: 9578424 Review. - Antibody-interleukin 2 fusion proteins: a new approach to cancer therapy.
Reisfeld RA, Gillies SD. Reisfeld RA, et al. J Clin Lab Anal. 1996;10(3):160-6. doi: 10.1002/(SICI)1098-2825(1996)10:3<160::AID-JCLA9>3.0.CO;2-F. J Clin Lab Anal. 1996. PMID: 8731505 Review. No abstract available.
Cited by
- Activation of Hepatic Stellate Cells During Liver Carcinogenesis Requires Fibrinogen/Integrin αvβ5 in Zebrafish.
Yan C, Yang Q, Gong Z. Yan C, et al. Neoplasia. 2018 May;20(5):533-542. doi: 10.1016/j.neo.2018.02.002. Epub 2018 Apr 9. Neoplasia. 2018. PMID: 29649779 Free PMC article. Retracted. - PPARgamma in Neuroblastoma.
Peri A, Cellai I, Benvenuti S, Luciani P, Baglioni S, Serio M. Peri A, et al. PPAR Res. 2008;2008:917815. doi: 10.1155/2008/917815. PPAR Res. 2008. PMID: 18528516 Free PMC article. - Targeting angiogenesis for controlling neuroblastoma.
Roy Choudhury S, Karmakar S, Banik NL, Ray SK. Roy Choudhury S, et al. J Oncol. 2012;2012:782020. doi: 10.1155/2012/782020. Epub 2011 Aug 25. J Oncol. 2012. PMID: 21876694 Free PMC article. - Chemically programmed monoclonal antibodies for cancer therapy: adaptor immunotherapy based on a covalent antibody catalyst.
Rader C, Sinha SC, Popkov M, Lerner RA, Barbas CF 3rd. Rader C, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5396-400. doi: 10.1073/pnas.0931308100. Epub 2003 Apr 17. Proc Natl Acad Sci U S A. 2003. PMID: 12702756 Free PMC article. - A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer.
Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, Hossfeld DK, Stöger H, Neyns B, Herzog P, Piedbois P, Dobrowolski F, Scheithauer W, Hawkins R, Katz F, Balcke P, Vermorken J, van Belle S, Davidson N, Esteve AA, Castellano D, Kleeff J, Tempia-Caliera AA, Kovar A, Nippgen J. Friess H, et al. BMC Cancer. 2006 Dec 11;6:285. doi: 10.1186/1471-2407-6-285. BMC Cancer. 2006. PMID: 17156477 Free PMC article. Clinical Trial.
References
- Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1995;1:149–153. - PubMed
- Folkman J. Nat Med. 1995;1:27–31. - PubMed
- O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. - PubMed
- Kerbel R S. Nature (London) 1997;390:335–336. - PubMed
- Boehm T, Folkman J, Browder T, O’Reilly M S. Nature (London) 1997;390:404–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 CA050286/CA/NCI NIH HHS/United States
- CA-50286/CA/NCI NIH HHS/United States
- R01 CA050286/CA/NCI NIH HHS/United States
- R01 CA045726/CA/NCI NIH HHS/United States
- CA-45726/CA/NCI NIH HHS/United States
- CA-42508/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases