Rutherford and the atom (1 of 4) - Understanding Science (original) (raw)
Image Caption
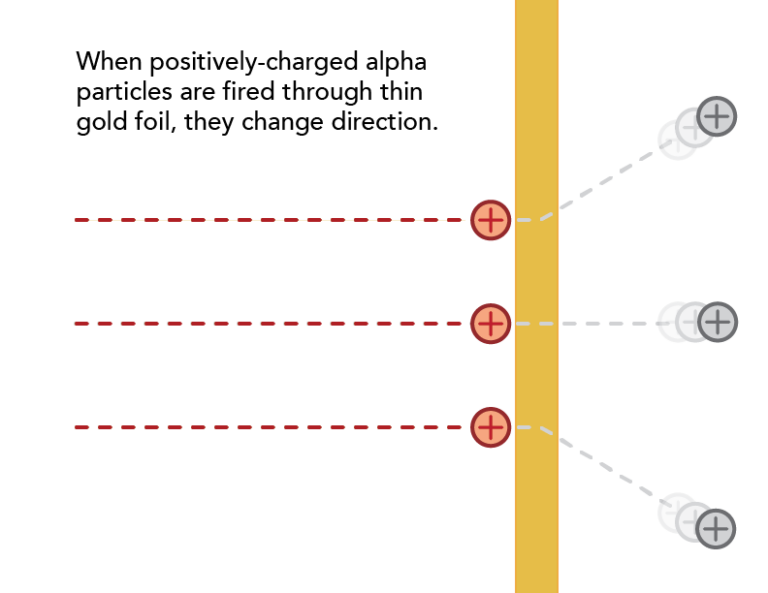
Ernest Rutherford used alpha particles (helium atoms stripped of their electrons) to learn about the structure of the atom.
Image use policy: For non-commercial, educational purposes, this image may be used with a Creative Commons CC BY-NC-SA 4.0 license. Please credit as follows: © University of California Museum of Paleontology, Understanding Science, www.understandingscience.org
See where this image appears on the Understanding Science website »
To save: 1) Click on image for the full-size version, 2) right-click (Windows) or control-click (Mac) on the image, and 3) select "Save image."
This image is part of a series:
Rutherford and the atom (2 of 4)
By firing alpha particles through gold foil, Rutherford was able to test ideas about the interior of the atom.
Rutherford and the atom (3 of 4)
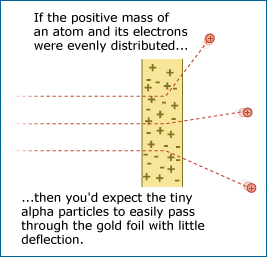
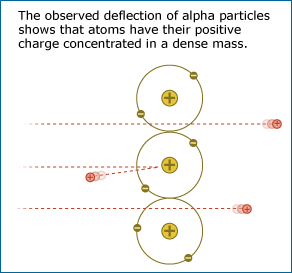
Most of the alpha particles passed through the gold foil without changing direction much as expected, but some came bouncing back in the opposite direction.
Rutherford and the atom (4 of 4)
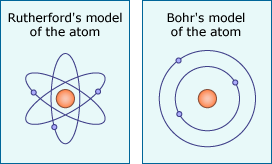
Rutherford published a description of his idea, which was later modified by Niels Bohr.