CO sensing mechanism with WO3 based gas sensors (original) (raw)
Abstract
The interaction between CO and WO 3 based gas sensors was investigated in operando conditions by using resistance, catalytic conversion and DRIFTS measurements. The experimental results are showing that the sensing involves the reduction of the metal oxide, which is the opposite of the case of SnO 2 , the other extremely relevant material for semiconducting oxides based gas sensors.
FAQs
AI
What explains the mechanism of CO detection by WO3 sensors?add
The study reveals that the CO detection in WO3 sensors likely involves the reduction of the material, utilizing lattice oxygen to produce CO2. This reduction reaction distinguishes its sensing mechanism from that of SnO2-based materials.
How does oxygen concentration affect WO3 sensor performance with CO exposure?add
The paper finds that increasing oxygen concentration to 150 ppm decreases sensor signals while increasing CO2 production. This indicates an equilibrium between oxygen vacancies' generation and cancellation influenced by the surrounding oxygen levels.
What experimental methods were used to analyze CO sensing in WO3?add
Conductance, catalytic conversion, and DRIFTS measurements were employed to investigate CO sensing performance in WO3. The study particularly assessed sensor responses under various oxygen background conditions, including almost total absence of oxygen.
What role do oxygen vacancies play in the sensing mechanism of WO3?add
The findings suggest that oxygen vacancies in WO3 are crucial for sensing, as their ionization decreases resistance during CO interaction. This interaction is essential in understanding the gas sensing mechanisms compared to SnO2 materials.
What are the unique contributions of WO3 compared to SnO2 in gas sensing?add
Unlike SnO2, WO3 sensors can produce CO2 even in low oxygen conditions, indicating a direct material reduction. This significant deviation in sensing behavior necessitates re-evaluating existing understanding of reducing gas interactions.
Figures (2)
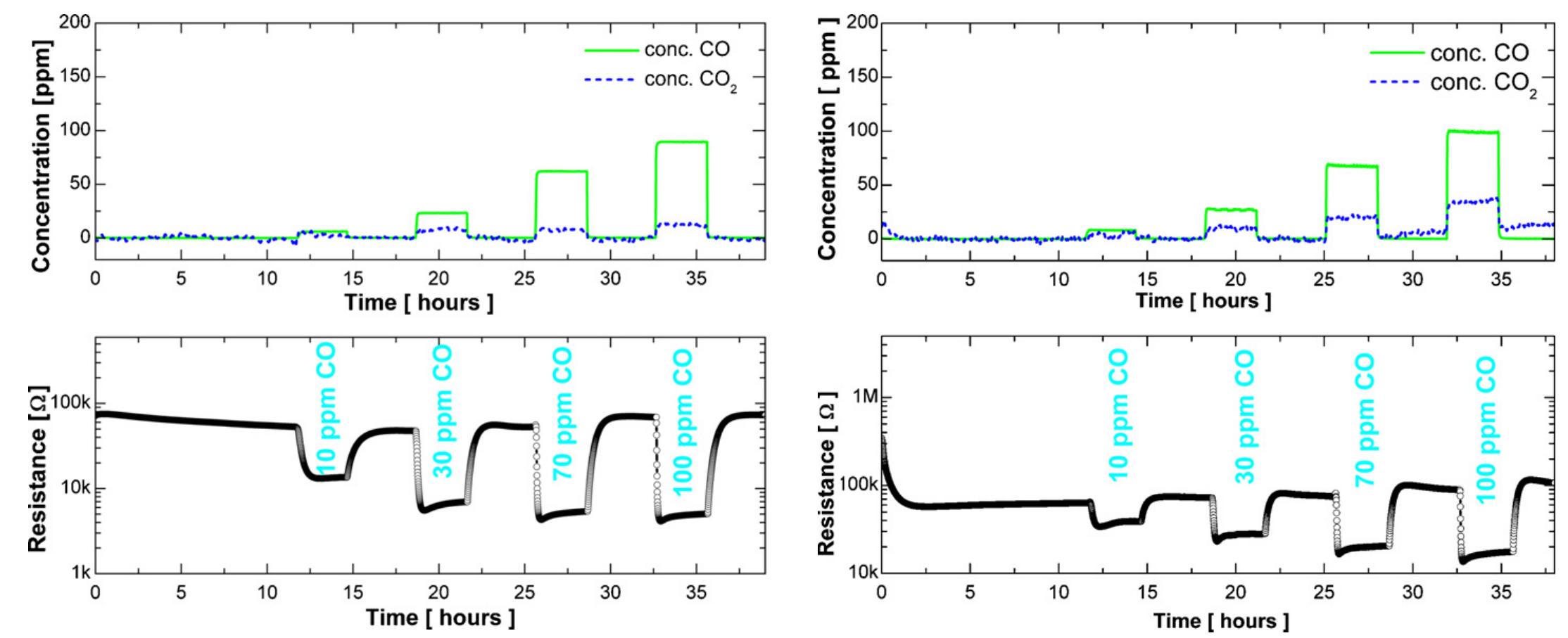
Fig. 1. Time dependence of the resistance during CO exposure (10, 30, 70, 100 ppm) in the absence of oxygen at 300°C. The amount of CO and CO; in the exhaust is shown in the upper part. Fig. 2. Time dependence of the resistance during CO exposure (10, 30, 70, 100 ppm) in the presence of 150 ppm of oxygen. The amount of CO and CO in the exhaust is shown in the upper part.
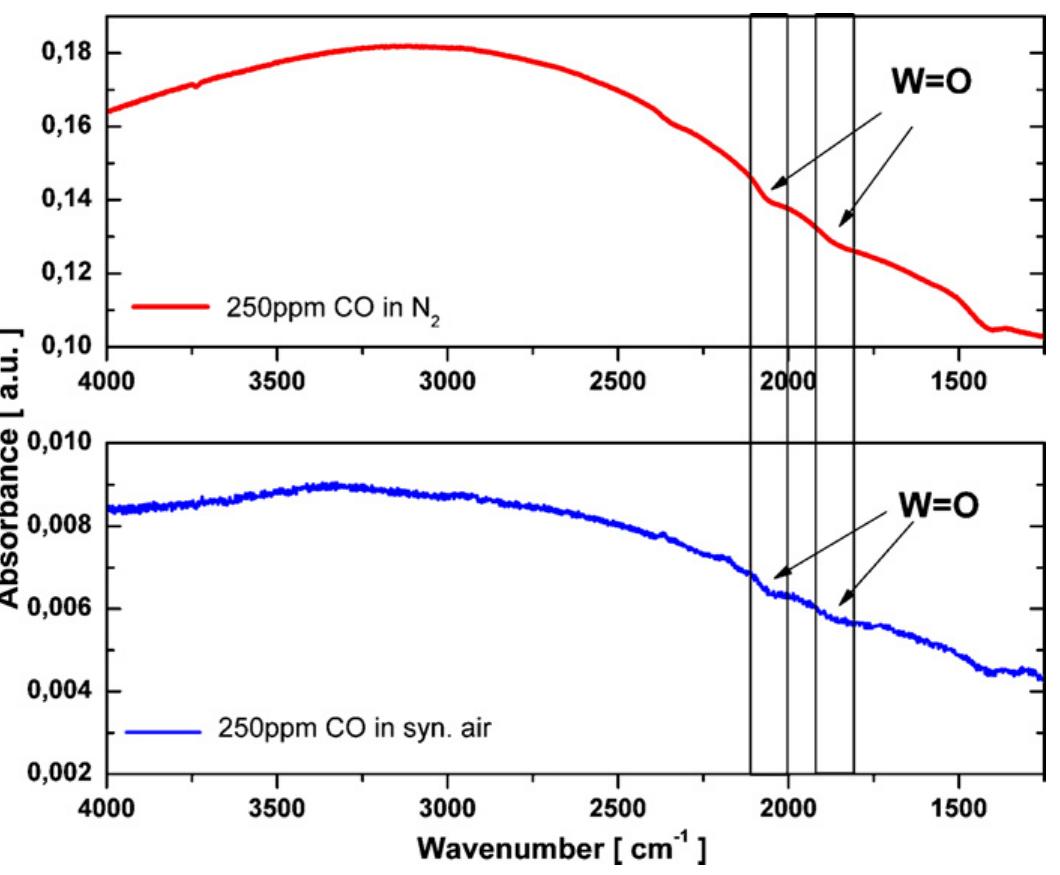
Fig. 3. DRIFT absorbance spectra for WO3 sensor in the absence of oxygen, in the upper part (red), and in dry synthetic air, in the lower part (blue), at 300°C during exposure to 250 ppm of CO. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (21)
- T. Inoue, K. Ohtsuka, Y. Yoshida, Y. Matsuura, Y. Kajiyama, Metal oxide semi- conductor NO2 sensor, Sensors and Actuators 25 (1-3) (1995) 388-391.
- T. Sauerwald, Investigation of surface processes affecting a multi signal gen- eration of tin oxide and tungsten oxide gas sensors, PhD thesis, Justus-Liebig- Universität Gießen http://geb.uni-giessen.de/geb/volltexte/2008/5500/.
- D. Briand, L. Guillot, S. Raible, J. Kappler, N.F. de Rooij, Highly Integrated Wafer Level Packaged MOX Gas Sensors, Digest of technical papers TRANS- DUCERS 2007 & Eurosensors XXI, Lyon, France ISBN 1-4244-0842-3, 2007, pp. 2401-2404.
- P.J. Shaver, Activated tungsten oxide gas detector, Applied Physics Letters 11 (1967) 255.
- M. Akiyama, J. Tamaki, N. Miura, N. Yamazoe, Tungsten oxide-based semi- conductor sensor highly sensitive to NO and NO2, Chemistry Letters (1991) 1611-1614.
- G. Sberveglieri, L. Depero, S. Groppeli, P. Nelli, WO3 sputtered thin films for NOx monitoring, Sensors and Actuators B 26-27 (1995) 89-92.
- T. Maekawa, J. Tamaki, N. Miura, N. Yamazoe, Gold-loaded tungsten-oxide sen- sor for detection of ammonia in air, Chemistry Letters 4 (1992) 639-642.
- H. Meixner, J. Gerblinger, U. Lampe, M. Fleischer, Thin-film gas sensors based on semiconducting metal oxides, Sensors and Actuators B 23 (2-3) (1995) 119-125.
- I. Ruokamo, T. Karkkainen, J. Huusko, T. Ruokanen, M. Blomberg, H. Torvela, V. Lantto, H2S response of WO3 thin film sensors manufactured by Silican processing technology, Sensors and Actuators B 19 (1-3) (1994) 486-488.
- Landolt, Börnstein: Numerical Data and Functional Relationship in Science and Technology, Volume 17 Semiconductors Subvolume g Physics of Non- tetrahedrally Bonded Binary Compounds II, Springer Verlag, Berlin New York, 1982, pp. 166-200, 446, 594.
- S.L. Baumann, Detektions-Mechanismen auf WO3 bei Einsatz in Verbren- nungsabgasen, PhD thesis, Justus-Liebig-Universität Gießen, http://geb.uni- giessen.de/geb/volltexte/2004/1432/.
- E. Llobet, G. Molas, P. Molinàs, J. Calderer, X. Vilanova, J. Brezmes, J.E. Sueiras, X. Correig, Fabrication of highly selective tungsten oxide ammonia sensors, Journal of the Electrochemical Society 147 (2) (2000) 776-779.
- D.J. Dwyer, Surface chemistry of gas sensors -H2S on WO3 films, Sensors and Actuators B 5 (1-4) (1991) 155-159.
- B. Frühberger, M. Grunze, D.J. Dwyer, H2S interaction with WO3, XPS studies: surface chemistry of H2S-sensitive tungsten oxide films, Actuators B 31 (3) (1996) 167-174.
- S.H. Hahn, N. Barsan, U. Weimar, S.G. Ejakov, J.H. Visser, R.E. Soltis, CO sensing with SnO2 thick film sensors: role of oxygen and water vapour, Thin Solid Films 436 (2003) 17-24.
- M. Hübner, R.G. Pavelko, N. Barsan, U. Weimar, Influence of oxygen back- grounds on hydrogen sensing with SnO2 nanomaterials, Sensors and Actuators B, Available online 1 February (in press).
- S. Pokhrel, C.E. Simion, V.S. Teodorescu, N. Barsan, U. Weimar, Synthesis, mechanism, and gas-sensing application of surfactant tailored tungsten oxide nanostructures, Advanced Functional Materials 19 (11) (2009) 1767-1774.
- J. Kappler, A. Tomescu, N. Barsan, U. Weimar, CO consumption of Pd doped SnO2 based sensors, Thin Solid Films 391 (2001) 186-191.
- S. Emiroglu, N. Barsan, U. Weimar, V. Hoffmann, In situ diffuse reflectance infrared spectroscopy study of CO adsorption on SnO2, Thin Solid Films 391 (2001) 176-185.
- S.M. Kanan, Z. Lu, J.K. Cox, G. Bernhardt, C.P. Tripp, Identification of surface sites on monoclinic WO3 powders by infrared spectroscopy, Langmuir 18 (2002) 1707-1712.
- G. Ramis, G. Busca, C. Cristiani, L. Lietti, P. Forzatti, F. Bregani, Characterization of tungsat-titania catalysts, Langmuir 8 (1992) 1744-1749.