Anuran tadpole assemblages in riparian areas of the Middle Paraná River, Argentina (original) (raw)
Abstract
Acute toxicity and genotoxicity of the S-metolachlor-based herbicide Dual Gold ® on Leptodactylus luctator (Hudson, 1892) tadpoles (Anura: Leptodactylidae). Herbicides used in agriculture and their metabolites are frequently detected in surface water bodies, where they can persist and cause adverse effects on aquatic organisms. The aim of this study was to evaluate the acute toxicity and genotoxic effects of the S-metolachlor (SM)-based herbicide Dual Gold ® (DG ®), on Leptodactylus luctator tadpoles (Anura: Leptodactylidae). To assess the toxicity of the herbicide, including the median lethal concentration (LC50) at 24h, the no-observed-effect concentration (NOEC), and the lowest-observed-effect concentration (LOEC), tadpoles were exposed to five nominal concentrations of DG ® (5.0, 6.2, 7.8, 9.8, and 12.2 mg/L), and to dechlorinated water as a negative control (NC). The LC50 24h of DG ® was 7.0 mg/L, the NOEC was 5.0 mg/L and the LOEC=6.2 mg/L. L. luctator tadpoles were sensitive to the herbicide, reaching 100% mortality after 24 h of exposure to the highest concentration tested (12.2 mg/L). To evaluate the potential genotoxicity of the herbicide, the frequencies of micronuclei (MN) and other erythrocyte nuclear abnormalities (ENA) were determined in larvae exposed to three nominal concentrations of DG ® (1.0, 5.0, and 6.2 mg/L) for 48 and 96 h. The frequencies of MN and ENA were compared with a positive control (40 mg/L of Cyclophosphamide) and a negative control. The frequencies of MN and ENA in the erythrocytes of tadpoles exposed to the test concentrations of DG ® and Cyclophosphamide were significantly higher than in the negative control group at both 48 and 96 h (with the only exception of MN at 1.0 mg/L at 48 h). Our results confirm the genotoxic and cytotoxic effects of this widely used herbicide in agriculture, a fact that represents a potential risk to amphibians that develop in ponds associated with or immersed in agroecosystems.
Figures (3)
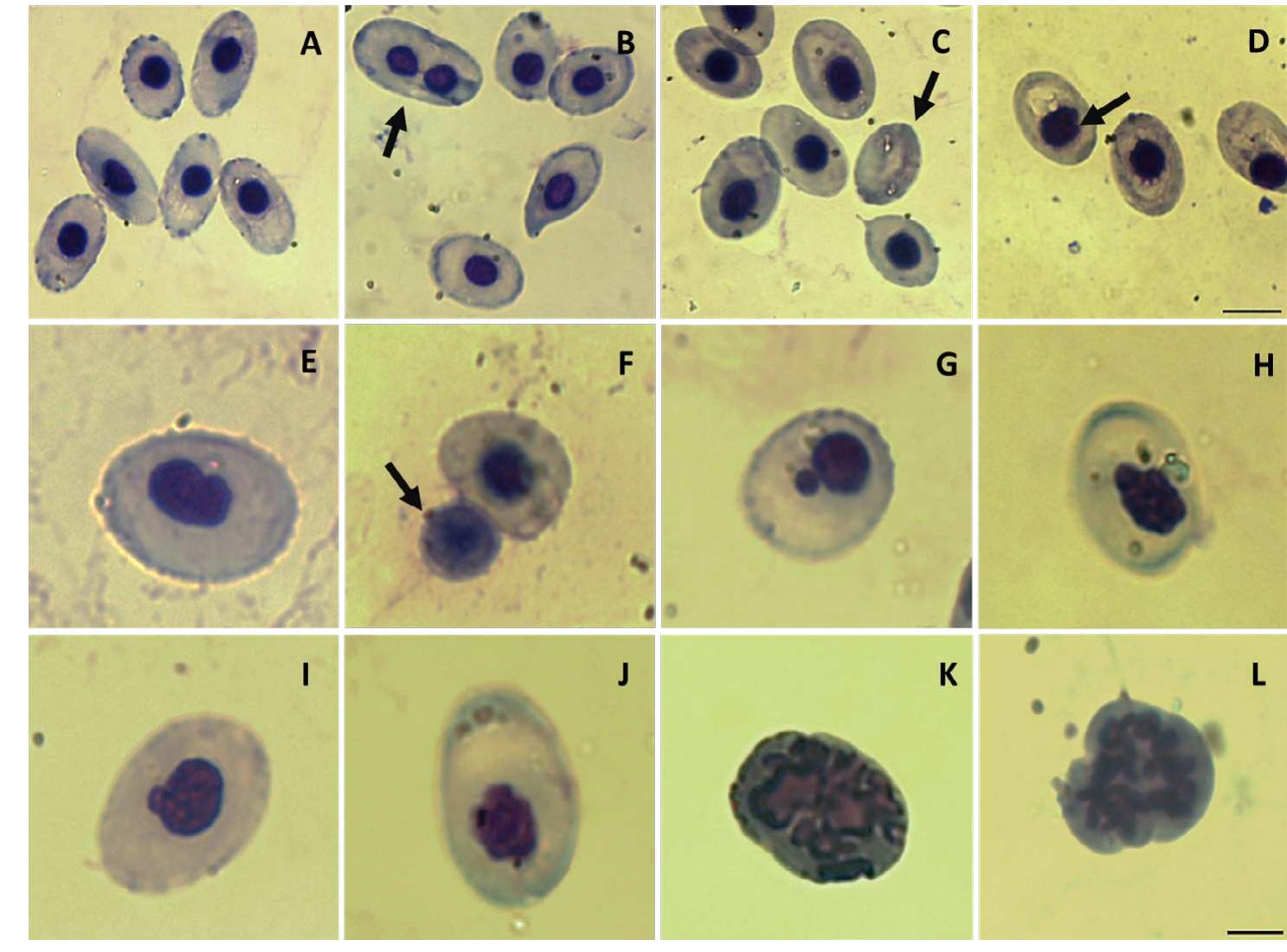
Figure 1. Details of erythrocytes observed in Leptodactylus luctator tadpoles exposed to the SM-based herbicide DG®. (A) Normal erythrocytes; (B) Binucleated erythrocyte (BE); (C) Erythroplastid or anucleated erythrocyte (EP); (D) Notched nucleus (NN); (E) Kidney-shaped nucleus (K); (F) Pyknotic nucleus (PN); (G) Micronucleus (MN); (H) Lobed nucleus (LN); (1) Budded nucleus (BN); (J) Blebbed nucleus (BbN); (K) Apoptotic cell (AP); (L) Erythrocyte in mitosis (M). May Griinwald-Giemsa staining, 100x. Black bar in image D represents a scale of 10 um (for images A-D). Black bar in image L represents a scale of 5 wm (for images E-L). Detalle de eritrocitos observados en larvas de Leptodactylus luctator expuestas al herbicida DG®, a base de SM. (A) eritrocitos normales, (B) eri- trocito binucleado (BE); (C) eritoplastido o eritrocito anucleado (EP); (D) nucleo con muesca (NN); (E) nucleo en forma de rifion (K); (F) nucleo picnotico (PN); (G) Microntcleo (MN); (H) nucleo lobado (LN); (1) nicleo con brote (BN); (J); nucleo ampollado (BbN); (K) célula apoptotica (AP); (L) eritrocito en mitosis (M). May Griinwald-Giemsa, 100x. La barra negra en la imagen D representa una escala de 10 um (imagenes A-D). La barra negra en la imagen L representa una escala de 5 um (imagenes E-L).
Figure 2. Frequency of micronuclei (MN) (per-1000 cells) in Leptodactylus luctator tadpoles exposed to different concentra- tions of the SM-based herbicide DG®. NC: negative control (0 mg/L of SM); PC: positive control (40 mg/L of cyclophospha- mide); Ta=1 mg/L of SM; Tb=5.0 of mg/L SM; Tc=6.2 mg/L of SM. RBCs= red blood cells. * p<0.05: significant differences compared to the negative control (binomial proportion test). Frecuencia de micronticleos (MN) (por 1000 células) en larvas de Leptodactylus luctator expuestas a diferentes concentracio- nes del herbicida DG®, a base de SM. NC: control negativo (0 mg/L de SM); CP: control positivo (40 mg/L de ciclofosfamida); Ta=1 mg/L de SM; Tb=5.0 de mg/L SM; Tc=6.2 mg/L de SM. *p<0.05: diferencias significativas con respecto al control ne- gativo (Test de proporcion binomial). Figure 3. Frequency of erythrocytes nuclear abnormalities (ENA) (per-1000 cells) in Leptodactylus luctator tadpoles ex- posed to different concentrations of SM-based herbicide DG®. NC: negative control (0 mg/L of SM); PC: positive control (40 mg/L of cyclophosphamide); Ta=1 mg/L of SM; Tb=5.0 mg/L of SM; Tc=6.2 mg/L of SM. RBCs= red blood cells. * p<0.05: sig- nificant differences compared to the negative control (binomial proportion test). Frecuencia de Aberraciones nucleares en los ertirocitos (ANE) (por 1000 células) en larvas de Leptodactylus luctator expuestas a diferentes concentraciones del herbicida DG®, a base de SM. NC: control negativo (0 mg/L de SM); CP: control positivo (40 mg/L de ciclofosfamida); Ta=1 mg/L de SM; Tb=5.0 mg/L de SM; Tce=6.2 mg/L de SM. *p<0.05: diferencias significativas con respecto al control negativo (Test de propor- cion binomial). Ecotoxicity of the herbicide Dual Gold® on Leptodactylus luctator tadpoles
Table 1. Frequency (%o) of different types of erythrocytes nuclear abnormalities (ENA) in L. /uctator tadpoles exposed to different concentrations of SM (mean + SE). NC: negative control (0 mg/L of SM); PC: positive control (40 mg/L of cyclo- phosphamide); Ta=1 mg/L of SM; Tb=5.0 mg/L of SM; Tc=6.2 mg/L of SM; BE: binucleated erythrocytes; K: kidney-shaped nuclei; EP: erythroplastids or anucleated erythrocytes; BN: budded nuclei; LN: lobed nuclei; BbN: blebbed nuclei; NN: notched nuclei; PN: pyknotic nuclei; AP: apoptotic cells. *p<0.05: significant differences compared to the negative control (binomial proportion test). Frecuencia (%o) de distintos tipos de aberraciones nucleares en eritrocitos (ANE) de larvas de L. luctator expuestas a diferentes concentraciones de SM (media + SE). NC: control negativo (0 mg/L de SM); CP: control posi- tivo (40 mg/L de ciclofosfamida); Ta=1 mg/L de SM; Tb=5.0 mg/L de SM; Tc=6.2 mg/L de SM; BE: eritrocitos binucleados; K: nucleos en forma de rion; EP: eritoplastidos o eritrocitos anucleados; BN: nicleos con brotes; LN: ntcleos lobados; BBN: nucleos ampollados; NN: nucleos con muescas; PN: nucleos picnoticos; AP: célula apoptotica. *p<0.05: diferencias significativas con respecto al control negativo (Test de proporcion binomial). mg/L has been reported for SM on embryos of the common frog Pelophylax perezi (Quintaneiro et al., 2018). Our results suggest that L. /uctator tad- poles are less tolerant to SM than P. perezi embry- os. Differences in sensitivity could be attributed to intrinsic differences between species, as well as to the different developmental stages evaluated, and to the commercial formulation used in this study, which, in addition to the active ingredient contains excipients such as surfactants and solvents, which may influence its toxicity. Previous studies have shown that a commercial formulation of various agrochemicals may be more toxic than the active ingredient itself (K6nen & Cava, 2008; Nikoloff et al., 2013; Lajmanovich et al., 2014; Bach et al., 2016). While DG® contains more than twice the amount of SM in its formulation than Primextra® II, it is not possible to directly compare the sensi-

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (82)
- Andrade, V. S., Gutierrez, M. F., Regaldo, L., Pai- ra, A. R., Repetti, M. R., & Gagneten, A. M. (2021). Influence of rainfall and seasonal crop practices on nutrient and pesticide runoff from soybean dominated agricultural areas in Pam- pean streams, Argentina. Science of the Total Environment, 788, 147676. DOI: 10.1016/j. scitotenv.2021.147676.
- Araújo, C. V., Shinn, C., Moreira-Santos, M., Lopes, I., Espíndola, E. L., & Ribeiro, R. (2014). Copper-driven avoidance and mortali- ty in temperate and tropical tadpoles. Aquatic toxicology, 146, 70-75. DOI: 10.1016/j.aqua- tox.2013.10.030.
- Attademo, A. M., Peltzer, P. M., & Lajmanovich, R. C. (2005). Amphibians occurring in soy- bean and implications for biological control in Argentina. Agriculture, ecosystems & en- vironment, 106(4), 389-394. DOI: 10.1016/j. agee.2004.08.012.
- Attademo, A. M., Peltzer, P. M., Lajmanovich, R. C., Cabagna-Zenklusen, M. C., Junges, C. M., & Basso, A. (2014). Biological endpoints, en- zyme activities, and blood cell parameters in two anuran tadpole species in rice agroecosys- tems of mid-eastern Argentina. Environmental monitoring and assessment, 186, 635-649. DOI: 10.1007/s10661-013-3404-z.
- Atwood, D., & Paisley-Jones, C. (2017). Pesti- cides industry sales and usage: 2008-2012 market estimates. US Environmental Protec- tion Agency, Washington, DC, 20460, 2017- 01.
- Ayres, M., Ayres, J. R. M., Ayres, D. L., & Santos, A. S. (2007). BioEstat 5.0-aplicações estatísti- cas nas áreas das ciências biológicas e médi- cas: Sociedade Civil Mamirauá, Belém. Bra- sília: CNPq.
- Bach, N. C., Natale, G. S., Somoza, G. M., & Ronco, A. E. (2016). Effect on the growth and development and induction of abnormalities by a glyphosate commercial formulation and its active ingredient during two developmen- tal stages of the South-American Creole frog, Leptodactylus latrans. Environmental Science and Pollution Research, 23, 23959-23971. DOI: 10.1007/s11356-016-7631-z.
- Bach, N. C., Marino, D. J., Natale, G. S., & So- moza, G. M. (2018). Effects of glyphosate and its commercial formulation, Roundup ® Ultra- max, on liver histology of tadpoles of the neo- tropical frog, Leptodactylus latrans (amphib- ia: Anura). Chemosphere, 202, 289-297. DOI: 10.1016/j.chemosphere.2018.03.110.
- Baeza, S., Vélez-Martin, E., De Abelleyra, D., Banchero, S., Gallego, F., Schirmbeck, J., ...
- & Hasenack, H. (2022). Two decades of land cover mapping in the Río de la Plata grassland region: The MapBiomas Pampa initiative. Remote Sensing Applications: Society and Environment, 28, 100834. DOI: 10.1016/j. rsase.2022.100834.
- Barni, S., Boncompagni, E., Grosso, A., Ber- tone, V., Freitas, I., Fasola, M., & Fenoglio, C. (2007). Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquatic Toxicology, 81(1), 45-54. DOI: 10.1016/j.aquatox.2006.10.012.
- Benvindo-Souza, M., Oliveira, E. A. S., Assis, R. A., Santos, C. G. A., Borges, R. E., e Silva, D. D. M., & de Souza Santos, L. R. (2020). Micronucleus test in tadpole erythrocytes: trends in studies and new paths. Chemo- sphere, 240, 124910. DOI: 10.1016/j.chemo- sphere.2019.124910.
- Bionda, C. D. L., Babini, S., Martino, A. L., Salas, N. E., & Lajmanovich, R. C. (2018). Impact assessment of agriculture and livestock over age, longevity and growth of populations of common toad Rhinella arenarum (anura: Bu- fonidae), central area of Argentina. Global Ecology and Conservation, 14, e00398. DOI: 10.1016/j.gecco.2018.e00398.
- Bolognesi, C., Perrone, E., Roggieri, P., Pampanin, D. M., & Sciutto, A. (2006). Assessment of mi- cronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquatic toxicology, 78, S93-S98. DOI: 10.1016/j.aquatox.2006.02.015.
- Carrasco, K. R., Tilbury, K. L., & Myers, M. S. (1990). Assessment of the piscine micronu- cleus test as an in situ biological indicator of chemical contaminant effects. Canadian Jour- nal of Fisheries and Aquatic Sciences, 47(11), 2123-2136. DOI: 10.1139/f90-237.
- Carreira, S. & Maneyro, R. (2015). Lista Roja de los Anfibios y Reptiles del Uruguay. Dirección Nacional de Medio Ambiente, Montevideo, Uruguay.
- Çavaş, T., & Ergene-Gözükara, S. (2005). Induc- tion of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium process- ing plant effluents. Aquatic toxicology, 74(3), 264-271. DOI: 0.1016/j.aquatox.2005.06.001.
- de Arcaute, C. R., Brodeur, J. C., Soloneski, S., & Larramendy, M. L. (2020). Toxicity to Rhi- nella arenarum tadpoles (Anura, Bufonidae) of herbicide mixtures commonly used to treat fallow containing resistant weeds: glyphosate- dicamba and glyphosate-flurochloridone.
- Chemosphere, 245, 125623. DOI: 10.1016/j. chemosphere.2019.125623.
- De Liguoro, M., Bona, M. D., Gallina, G., Cap- olongo, F., Gallocchio, F., Binato, G., & Di Leva, V. (2014). A monitoring of chemical contaminants in waters used for field irriga- tion and livestock watering in the Veneto re- gion (Italy), using bioassays as a screening tool. Environmental Science and Pollution Re- search, 21, 3546-3557. DOI: 10.1007/s11356- 013-2357-7.
- Eterovick, P. C., Sloss, B. L., Scalzo, J. A., & Al- ford, R. A. (2016). Isolated frogs in a crowded world: Effects of human-caused habitat loss on frog heterozygosity and fluctuating asym- metry. Biological Conservation, 195, 52-59. DOI: 10.1016/j.biocon.2015.12.036.
- Fenech, M. (2000). The in vitro micronucleus technique. Mutation Research/Fundamental and Molecular Mechanisms of Mutagene- sis, 455(1-2), 81-95. DOI: 10.1016/S0027- 5107(00)00065-8.
- Freitas, J. S., Girotto, L., Goulart, B. V., Alho, L. D. O. G., Gebara, R. C., Montagner, C. C., ... & Espíndola, E. L. G. (2019). Effects of 2, 4-D-based herbicide (DMA ® 806) on sensitivity, respiration rates, energy reserves and behavior of tadpoles. Ecotoxicology and environmental safety, 182, 109446. DOI: 10.1016/j.ecoenv.2019.109446.
- Gilliom, R. J., Barbash, J. E., Crawford, C. G., Hamilton, P. A., Martin, J. D., Nakagaki, N., …. Wolock, D. M. (2006). Pesticides in the nation's streams and ground water, 1992-2001: U.S. Geological Survey Circular 1291, The Quality of Our Nation's Waters. https://pubs.er.usgs.gov/publication/cir1291\. DOI:10.3133/cir1291.
- Guilherme, S., Válega, M., Pereira, M. E., Santos, M. A., & Pacheco, M. (2008). Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercu- ry contamination gradient. Ecotoxicology and Environmental Safety, 70(3), 411-421. DOI: 10.1016/j.ecoenv.2007.08.016.
- Gosner, K. L. (1960). A simplified table for stag- ing anuran embryos and larvae with notes on identification. Herpetologica, 16(3), 183-190.
- Gripp, H. S., Freitas, J. S., Almeida, E. A., Bisino- ti, M. C., & Moreira, A. B. (2017). Biochem- ical effects of fipronil and its metabolites on lipid peroxidation and enzymatic antioxidant defense in tadpoles (Eupemphix nattereri: Leiuperidae). Ecotoxicology and environmen- tal safety, 136, 173-179. DOI: 10.1016/j.eco- env.2016.10.027.
- Hamilton, M. A., Russo, R. C., & Thurston, R. V. (1977). Trimmed Spearman-Karber meth- od for estimating median lethal concentrations in toxicity bioassays. Environmental science & technology, 11(7), 714-719. DOI: 10.1021/ es60130a004.
- Hammer, Ø., Harper, D. A., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Pa- laeontologia electronica, 4(1), 9.
- Heyer, R., Langone, J., La Marca, E., Azeve- do-Ramos, C., di Tada, I., Baldo, D., ... & Hardy, J. (2010). Leptodactylus latrans. The IUCN Red List of Threatened Species 2010: e. T57151A11592655. http://www.iucnredlist. org. Accessed on 23 February 2023. IUCN 2022. The IUCN Red List of Threatened Species. Version 2021-3. http://www.iucnred- list.org. Accessed on 23 February 2023.
- Josende, M. E., Tozetti, A. M., Alalan, M. T., Ma- thies Filho, V., da Silva Ximenez, S., da Sil- va Júnior, F. M. R., & Martins, S. E. (2015). Genotoxic evaluation in two amphibian spe- cies from Brazilian subtropical wetlands. Eco- logical Indicators, 49, 83-87. DOI: 10.1016/j. ecolind.2014.10.007.
- Könen S., & Çavaş T. (2008). Genotoxicity test- ing of the herbicide trifluralin and its commer- cial formulation Treflan using the piscine mi- cronucleus test. Environmental and Molecular Mutagenesis, 49, 434-438. DOI: 10.1002/ em.20401.
- Lajmanovich, R. C., Cabagna, M., Peltzer, P. M., Stringhini, G. A., & Attademo, A. M. (2005). Micronucleus induction in erythrocytes of the Hyla pulchella tadpoles (Amphibia: Hylidae) exposed to insecticide endosulfan. Mutation Research/Genetic Toxicology and Environ- mental Mutagenesis, 587(1-2), 67-72. DOI: 10.1016/j.mrgentox.2005.08.001.
- Lajmanovich, R. C., Cabagna-Zenklusen, M. C., Attademo, A. M., Junges, C. M., Peltzer, P. M., Bassó, A., & Lorenzatti, E. (2014). Induc- tion of micronuclei and nuclear abnormalities in tadpoles of the common toad (Rhinella are- narum) treated with the herbicides Liberty® and glufosinate-ammonium. Mutation Re- search/Genetic Toxicology and Environmen- tal Mutagenesis, 769, 7-12. DOI: 10.1016/j. mrgentox.2014.04.009.
- Lajmanovich, R. C., Junges, C. M., Cabagna-Zen- klusen, M. C., Attademo, A. M., Peltzer, P. M., Maglianese, M., ... & Beccaria, A. J. (2015). Toxicity of Bacillus thuringiensis var. israel- ensis in aqueous suspension on the South American common frog Leptodactylus la- trans (Anura: Leptodactylidae) tadpoles. En- vironmental Research, 136, 205-212. DOI: 10.1016/j.envres.2014.10.022.
- Lajmanovich, R. C., Peltzer, P. M., Martinuzzi, C. S., Attademo, A. M., Colussi, C. L., & Basso, A. (2018). Acute toxicity of colloidal silicon dioxide nanoparticles on amphibian larvae: emerging environmental concern. Interna- tional Journal of Environmental Research, 12, 269-278. DOI: 10.1007/s41742-018-0089-8.
- Lajmanovich, R. C., Cuzziol Boccioni, A. P., Curi, L. M., Attademo, A. M., Martinuzzi, C., Bassó, A., Colussi, C., & Peltzer, P. M. (2021). Técnicas para el relevamiento de anfibios en ambientes contaminados. Pereyra, L., Etchepare, E., & Vaira, M (eds.): In: Manual de técnicas y protocolos para el relevamiento y estudio de anfibios de Argentina. (pp. 326-347). Universidad Nacional de Jujuy, Argentina.
- Lajmanovich, R. C., Attademo, A. M., Lener, G., Boccioni, A. P. C., Peltzer, P. M., Martinuzzi, C. S., ... & Repetti, M. R. (2022). Glyphosate and glufosinate ammonium, herbicides com- monly used on genetically modified crops, and their interaction with microplastics: Ecotoxic- ity in anuran tadpoles. Science of The Total Environment, 804, 150177. DOI: 10.1016/j. scitotenv.2021.150177.
- Lajmanovich, R. C., Repetti, M. R., Boccio- ni, A. P. C., Michlig, M. P., Demonte, L., Attademo, A. M., & Peltzer, P. M. (2023). Cocktails of pesticide residues in Prochilo- dus lineatus fish of the Salado River (South America): First record of high concentrations of polar herbicides. Science of The Total Envi- ronment, 870, 162019. DOI: 10.1016/j.scito- tenv.2023.162019.
- Lewis, K. A., Tzilivakis, J., Warner, D. J., & Green, A. (2016). An international database for pesticide risk assessments and manage- ment. Human and Ecological Risk Assess- ment: An International Journal, 22(4), 1050- 1064. DOI: 10.1080/10807039.2015.1133242.
- Liu, H., Ye, W., Zhan, X., & Liu, W. (2006). A comparative study of rac-and S-metolachlor toxicity to Daphnia magna. Ecotoxicology and Environmental Safety, 63(3), 451-455. DOI: 10.1016/j.ecoenv.2005.02.002.
- Liu, S., Wang, L., Chen, K., Yang, H., Ling, M., Wu, L., ... & Bai, L. (2022). Combined effects of S-metolachlor and benoxacor on embryo development in zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 238, 113565. DOI: 10.1016/j. ecoenv.2022.113565.
- Magalhães, F. D. M., Lyra, M. L., De Carvalho, T. R., Baldo, D., Brusquetti, F., Burella, P., ... & Garda, A. A. (2020). Taxonomic review of South American Butter Frogs: Phylogeny, geographic patterns, and species delimitation in the Leptodactylus latrans species group (Anura: Leptodactylidae). Herpetological Monographs, 34(1), 131-177. https://pubs. er.usgs.gov/publication/cir1291
- Mai, H., Morin, B., Pardon, P., Gonzalez, P., Budz- inski, H., & Cachot, J. (2013). Environmental concentrations of irgarol, diuron and S-meto- lachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas. Marine Environmental Research, 89, 1-8. DOI: 10.1016/j.marenvres.2013.04.003.
- Maneyro R. & Carreira S. (2012). Guía de an- fibios del Uruguay. 2da ed, Colección Cien- cia amiga-Ediciones de la Fuga. Montevideo, Uruguay.
- Margolin, B. H., Collings, B. J., & Mason, J. M. (1983). Statistical analysis and sample-size determinations for mutagenicity experiments with binomial responses. Environmental mutagenesis, 5(5), 705-716. DOI: 10.1002/ em.2860050509.
- McDaniel, T. V., Martin, P. A., Struger, J., Sher- ry, J., Marvin, C. H., McMaster, M. E., ... & Tetreault, G. (2008). Potential endocrine dis- ruption of sexual development in free ranging male northern leopard frogs (Rana pipiens) and green frogs (Rana clamitans) from areas of intensive row crop agriculture. Aquatic Tox- icology, 88(4), 230-242. DOI: 10.1016/j.aqua- tox.2008.05.002.
- Meintières, S., Biola, A., Pallardy, M., & Marz- in, D. (2001). Apoptosis can be a confusing factor in in vitro clastogenic assays. Mutage- nesis, 16(3), 243-250. DOI: 10.1093/muta- ge/16.3.243.
- Nardo, D., Evia, G., Castiglioni, E., Egaña, E., Galietta, G., Laporta, M., & Chichet, M. E. N. (2015). Determinación de glifosato medi- ante inmunoensayo enzimático (ELISA) en el paisaje protegido Laguna de Rocha su entor- no, Uruguay. Innotec, (10), 64-70.
- Nikoloff, N., Escobar, L., Soloneski, S., & Lar- ramendy, M. L. (2013). Comparative study of cytotoxic and genotoxic effects induced by herbicide S-metolachlor and its commer- cial formulation Twin Pack Gold ® in human hepatoma (HepG2) cells. Food and chemi- cal toxicology, 62, 777-781. DOI: 10.1016/j. fct.2013.10.015.
- Observatorio Ambiental Nacional. 2022. Datos abiertos. Datos propios y de otras instituciones vinculadas a la gestión ambiental. Ministerio de Ambiente. Montevideo, Uruguay. https:// pubs.er.usgs.gov/publication/cir1291. Accessed on 24 November 2022.
- Osano, O., Admiraal, W., & Otieno, D. (2002). Developmental disorders in embryos of the frog Xenopus laevis induced by chloroacet- anilide herbicides and their degradation prod- ucts. Environmental Toxicology and Chemis- try: An International Journal, 21(2), 375-379. DOI: 10.1002/etc.5620210221.
- Ossana, N. A., Castañé, P. M., & Salibián, A. (2013). Use of Lithobates catesbeianus tad- poles in a multiple biomarker approach for the assessment of water quality of the Reconquista River (Argentina). Archives of environmental contamination and toxicology, 65, 486-497. DOI: 10.1007/s00244-013-9920-6.
- PAN Pesticide Database. (2022). Metolachlor, (S). Pesticide Action Network, North America. https://pubs.er.usgs.gov/publication/cir1291, Accessed on 24 January 2023.
- Paunescu, A., Soare, L. C., Fierascu, R. C., Fier- ascu, I., & Ponepal, M. C. (2018). The influ- ence of six pesticides on physiological indices of Pelophylax ridibundus (Pallas, 1771). Bul- letin of environmental contamination and tox- icology, 100, 376-383. DOI: 10.1007/s00128- 018-2277-9.
- Peltzer, P. M., Lajmanovich, R. C., Attademo, A. M., Junges, C. M., Cabagna-Zenklusen, M. C., Repetti, M. R., ... & Beldoménico, H. (2013). Effect of exposure to contaminated pond sed- iments on survival, development, and enzyme and blood biomarkers in veined treefrog (Tra- chycephalus typhonius) tadpoles. Ecotoxicol- ogy and environmental safety, 98, 142-151. DOI: 10.1016/j.ecoenv.2013.09.010.
- Pérez-Iglesias, J. M., Soloneski, S., Nikoloff, N., Natale, G. S., & Larramendy, M. L. (2015). Toxic and genotoxic effects of the imazetha- pyr-based herbicide formulation Pivot H ® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicology and environmental safety, 119, 15-24. DOI: 10.1016/j.ecoenv.2015.04.045.
- Pérez-Iglesias, J. M., Franco-Belussi, L., Natale, G. S., & de Oliveira, C. (2019). Biomarkers at different levels of organisation after atrazine formulation (SIPTRAN 500SC ® ) exposure in Rhinella schineideri (Anura: Bufonidae) Neotropical tadpoles. Environmental pol- lution, 244, 733-746. DOI: 10.1016/j.en- vpol.2018.10.073.
- Pollo, F. E., Bionda, C. L., Salinas, Z. A., Salas, N. E., & Martino, A. L. (2015). Common toad Rhinella arenarum (Hensel, 1867) and its im- portance in assessing environmental health: test of micronuclei and nuclear abnormali- ties in erythrocytes. Environmental monito- ring and assessment, 187, 1-9. DOI:10.1007/ s10661-015-4802-1.
- Quintaneiro, C., Patrício, D., Novais, S. C., Soa- res, A. M. V. M., & Monteiro, M. S. (2017). Endocrine and physiological effects of li- nuron and S-metolachlor in zebrafish de- veloping embryos. Science of the Total En- vironment, 586, 390-400. DOI: 10.1016/j. scitotenv.2016.11.153.
- Quintaneiro, C., Soares, A. M., & Monteiro, M. S. (2018). Effects of the herbicides linuron and S-metolachlor on Perez's frog embryos. Che- mosphere, 194, 595-601. DOI: 10.1016/j.che- mosphere.2017.11.171.
- R Core Team. (2022). A language and environ- ment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed on 23 March 2022.
- Ray, M. R., Basu, C., Mukherjee, S., Roychow- dhury, S., & Lahiri, T. (2005). Micronucleus frequencies and nuclear anomalies in exfoliat- ed buccal epithelial cells of firefighters. Inter- national Journal of Human Genetics, 5(1), 45- 48. DOI: 10.1080/09723757.2005.11885915.
- Saccol, S. D. S. A., Bolzan, A. M. R., & dos San- tos, T. G. (2017). In the shadow of trees: does eucalyptus afforestation reduce herpetofaunal diversity in Southern Brazil?. South American Journal of Herpetology, 12(1), 42-56. DOI: 10.2994/SAJH-D-16-00028.1.
- Samojeden, C. G., Pavan, F. A., Rutkoski, C. F., Folador, A., da Fré, S. P., Müller, C., ... & Hart- mann, M. (2022). Toxicity and genotoxicity of imidacloprid in the tadpoles of Leptodactylus luctator and Physalaemus cuvieri (Anura: Leptodactylidae). Scientific Reports, 12(1), 11926. DOI: 10.1038/s41598-022-16039-z.
- Schmid W. (1975). The micronucleus test. Mu- tation Research, 31,9-15. DOI: https://pubs. er.usgs.gov/publication/cir1291.
- Serrano-García, L., & Montero-Montoya, R. (2001). Micronuclei and chromatid buds are the result of related genotoxic events. Envi- ronmental and molecular mutagenesis, 38(1), 38-45. DOI: 10.1002/em.1048.
- Shaner, D. L., Brunk, G., Belles, D., Westra, P., & Nissen, S. (2006). Soil dissipation and biolog- ical activity of metolachlor and S-metolachlor in five soils. Pest Management Science: for- merly Pesticide Science, 62(7), 617-623. DOI: 10.1002/ps.1215.
- Stara, A., Kubec, J., Zuskova, E., Buric, M., Fag- gio, C., Kouba, A., & Velisek, J. (2019). Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procam- barus virginalis). Chemosphere, 224, 616-625. DOI: 10.1016/j.chemosphere.2019.02.187.
- Tolbert, P. E., Shy, C. M., & Allen, J. W. (1992). Micronuclei and other nuclear anomalies in buccal smears: methods development. Mu- tation Research/Environmental Mutagenesis and Related Subjects, 271(1), 69-77. DOI: 10.1016/0165-1161(92)90033-I.
- Unrine, J. M., Hopkins, W. A., Romanek, C. S., & Jackson, B. P. (2007). Bioaccumulation of trace elements in omnivorous amphibian lar- vae: implications for amphibian health and contaminant transport. Environmental Pol- lution, 149(2), 182-192. DOI: 10.1016/j.en- vpol.2007.01.039
- USEPA. 1975. USEPA 660/375-009. Methods for acute toxicity tests with fish, macroinver- tebrates, and amphibians. United States Envi- ronmental Protection Agency. Manual. Wash- ington, D.C., EUA.
- Vallotton, N., Moser, D., Eggen, R. I., Junghans, M., & Chèvre, N. (2008). S-metolachlor pulse exposure on the alga Scenedesmus vacuolatus: effects during exposure and the subsequent re- covery. Chemosphere, 73(3), 395-400. DOI: 10.1016/j.chemosphere.2008.05.039.
- Van Opstal, N. V., Gabioud, E. A., Seehaus, M. S., Pighini, R. J., Repetti, M. R., Wilson, M. G., ...
- & Sasal, M. C. (2023). Spatial distribution of pesticides in surface water of the Estacas stream (Argentine Espinal region) associated with crop production. Environmental Science and Pollution Research, 30(15), 43573-43585. DOI: 10.1007/s11356-023-25373-2.
- Velisek, J., Stara, A., Zuskova, E., Kubec, J., Buric, M., & Kouba, A. (2019). Effects of s-metolachlor on early life stages of mar- bled crayfish. Pesticide biochemistry and physiology, 153, 87-94. DOI: 10.1016/j.pest- bp.2018.11.007.
- Vera-Candioti, J., Araujo, P. I., Huerga, I. R., Rojas, D. E., Cristos, D. S., & Malmantile, A. D. (2021). Pesticides detected in surface and groundwater from agroecosystems in the Pampas region of Argentina: occurrence and ecological risk assessment. Environmental Monitoring and Assessment, 193, 1-20. DOI: 10.1007/s10661-021-09462-8.
- Wan, M. T., Buday, C., Schroeder, G., Kuo, J., & Pasternak, J. (2006). Toxicity to Daph- nia magna, Hyalella azteca, Oncorhynchus kisutch, Oncorhynchus mykiss, Oncorhynchus tshawytscha, and Rana catesbeiana of atra- zine, metolachlor, simazine, and their formu- lated products. Bulletin of environmental con- tamination and toxicology, 76(1), 52-58. DOI: 10.1007/s00128-005-0888-4.
- Wells, K.D. (2007). The Ecology and Behavior of Amphibians. The University of Chicago Press.
- Williams, B. K., & Semlitsch, R. D. (2010). Lar- val responses of three Midwestern anurans to chronic, low-dose exposures of four her- bicides. Archives of Environmental Contam- ination and Toxicology, 58, 819-827. DOI: 10.1007/s00244-009-9390-z.
- Zhelev, Z., Tsonev, S., Georgieva, K., & Arnau- dova, D. (2018). Health status of Pelophylax ridibundus (Amphibia: Ranidae) in a rice paddy ecosystem in Southern Bulgaria and its importance in assessing environmental state: haematological parameters. Environmental Science and Pollution Research, 25, 7884- 7895. DOI: 10.1007/s11356-017-1109-5
- Zhu, X. W., & Chen, J. Y. (2016). mixtox: An R Package for Mixture Toxicity Assessment. R J., 8(2), 421.