What Is the Minimum Clinically Important Change in Negative Symptoms of Schizophrenia? PANSS Based Post-hoc Analyses of a Phase III Clinical Trial (original) (raw)
Abstract
IntroductionMinimum clinically important difference (MCID) is a measure that defines the minimum amount of change in an objective score of a clinical test that must be reached for that change to be clinically noticeable. We aimed to find the MCID for patients with predominantly negative symptoms of schizophrenia at its earliest occurrence.MethodsData of a 26-week long, double-blind study with 454 patients [Positive and Negative Symptom Scale Negative Factor Score (PANSS-FSNS) ≥24, Positive and Negative Symptom Scale Positive Factor Score (PANSS-FSPS) ≤ 19] treated with cariprazine 4.5 mg/d or risperidone 4 mg/d were analyzed. The Clinical Global Impression—Improvement scale was used to quantify minimum improvement (CGI-I = 3) and no clinical change (CGI-I = 4) on the PANSS-FSNS, and the MCID was estimated with the following methods: as the mean PANSS-FSNS changes corresponding to the first instance of minimal improvement across all visits (MCID1); as the difference between the PANSS...
Figures (4)
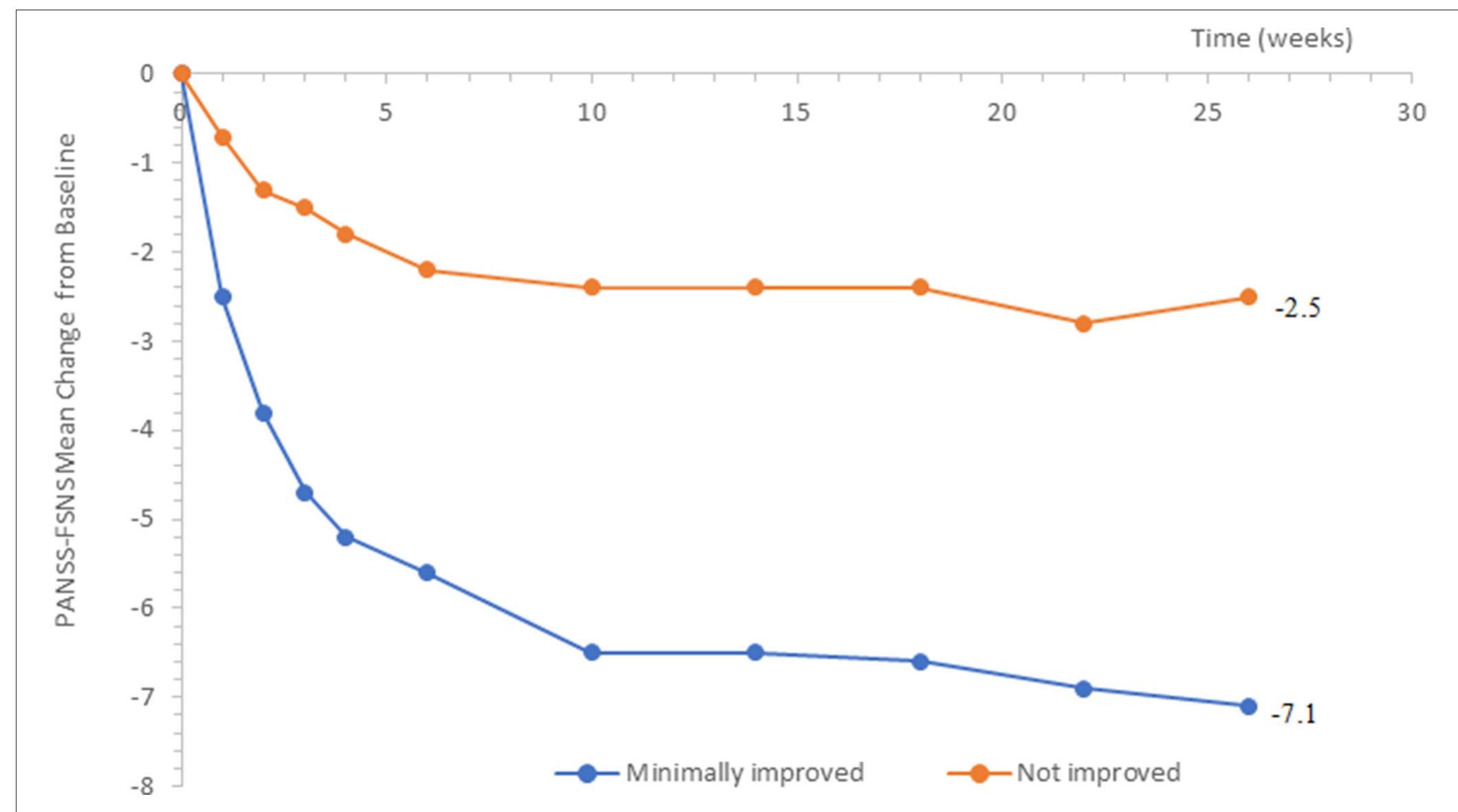
FIGURE 1 | Change from baseline in PANSS-FSNS (Positive- and Negative Syndrome Scale—Factor Score for Negative Symptoms) as a function of minimal change vs. no change.
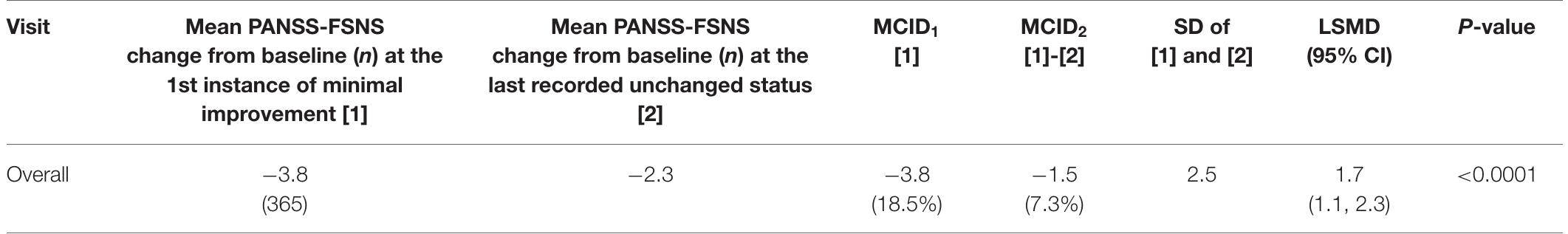
TABLE 1 | Anchor based calculations of the MCID.
FIGURE 2 | Receiver Operating Characteristic (ROC) curve: predictive accuracy of the PANSS-FSNS (Positive and Negative Syndrome Scale Factor Score for Negative Symptoms) scale for differentiating minimally improved vs. clinically unchanged status. The values on the vertical and horizontal axis, respectively, depict the sensitivity (“true positive rate”) and 1— specificity (“false positive rate”) values for the differentiation as a function of change from baseline in the PANSS-FSNS scale. The leftmost part of the ROC curve represents the highest empirically observed improvements in the sample as compared to baseline while the rightmost part represents no improvement (or even deterioration). Please note that the ROC curve for differentiating the minimally improved from the clinically unchanged status based on the PANSS-FSNS (ROC model, depicted in blue in the figure) significantly outperforms the random classification (ROC1 model, in red), with an area under the curve (AUG, labeled as “Area”) value of 0.7232 vs. 0.5000 (0 < 0.0001). PANSS-FSNS, Positive and Negative Syndrome Scale-Factor Score for Negative Symptoms; n, number of events; CGI-I, Clinical Global Impression—Improvement; MCID, and MCIDo, Minimum clinically important differences according to the definitions in the text; SD, Standard deviation; LSMD, Least-square mean difference between [1] and [2], as estimated by MMR\M, with corresponding 95% confidence limits. Please also note that for the computation of % changes from baseline the value of 7 was subtracted from the observed baseline severity (i.e., 27.6) since the minimum value of the PANSS-FSNS factor is 7 (i.e., the symptoms on all seven constituting items of the factor are rated as “Absent’).
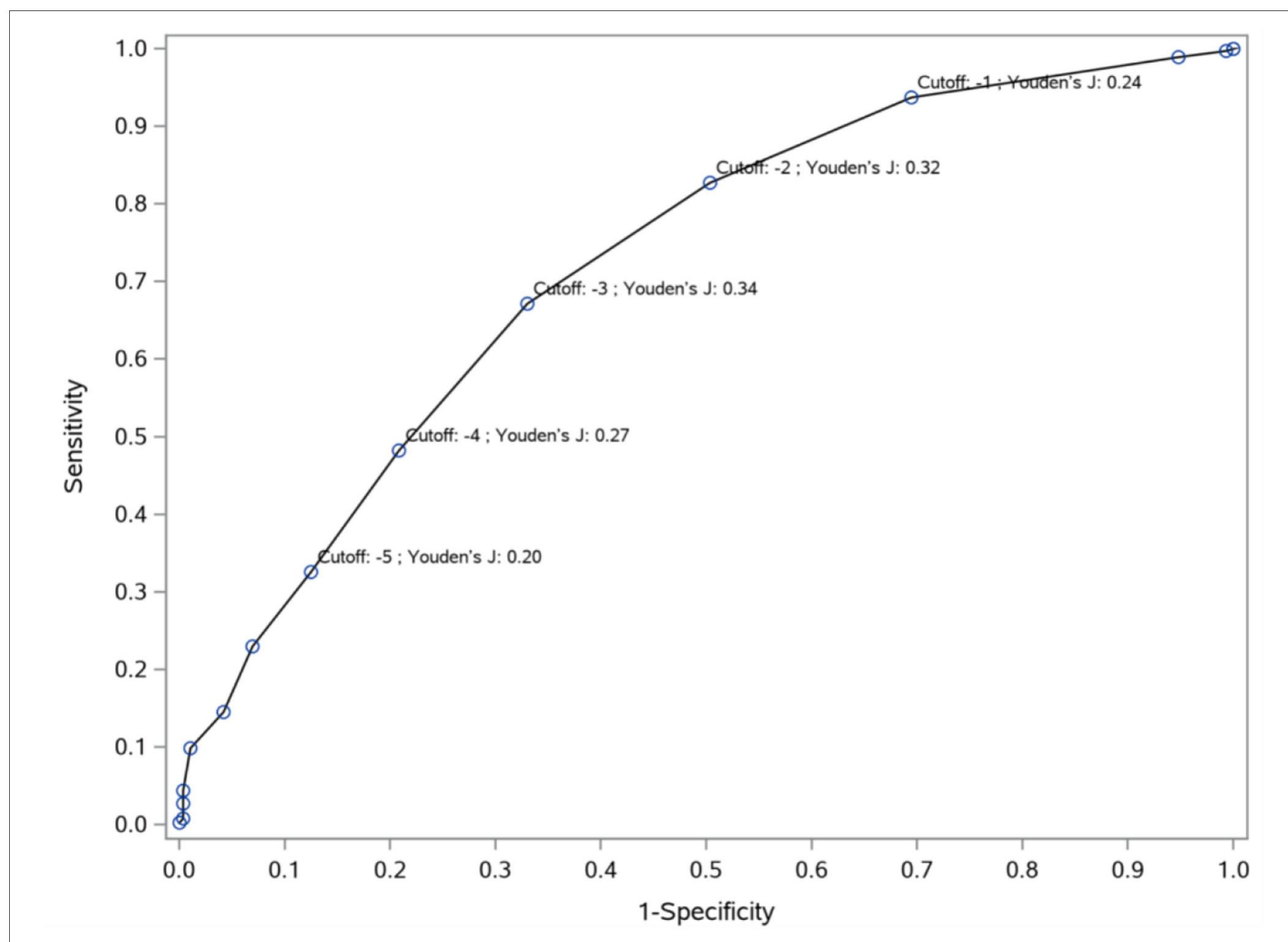
FIGURE 3 | Cut-off values (Youden’s indices) for predicting improvement from no clinical change to minimal improvement. To differentiate minimal improvement from no clinical change, Youden’s J indices were computed at different cut-off points based on the PANSS-FSNS change. The sensitivity (vertical axis) and 1- specificity (horizontal axis) values for the differentiation of minimal improvement from no clinical change are depicted in the figure for various values of change from baseline in th PANSS-FSNS (labeled as “Cutoff’). Please note that the Youden’s J index, which shows the efficiency of differentiation based on the combination of sensitivity and specificity, first increases then decreases with increasingly greater improvements (i.e., with greater negative values) as compared to baseline. The highest value of the Youden’s J Index is reached at the cut-off value of —3 (i.e., at a 3 point reduction of symptom severity from baseline in the PANSS-FSNS), which identifies the optima change value that maximizes sensitivity and specificity simultaneously.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (35)
- Correll CU, Kishimoto T, Nielsen J, Kane JM. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. (2011) 33:B16- 39. doi: 10.1016/j.clinthera.2011.11.016
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. (1989) 10:407-15. doi: 10.1016/0197-2456(89)90005-6
- Ferreira ML, Herbert RD, Ferreira PH, Latimer J, Ostelo RW, Nascimento DP, et al. A critical review of methods used to determine the smallest worthwhile effect of interventions for low back pain. J Clin Epidemiol. (2012) 65:253- 61. doi: 10.1016/j.jclinepi.2011.06.018
- Furukawa TA. Measuring clinical importance in a trial of interventions for mixed urinary incontinence. J Am Med Assoc. (2020) 323:479. doi: 10.1001/jama.2019.19730
- Beurskens AJHM, De Vet HCW, Köke AJA. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. (1996) 65:71-6. doi: 10.1016/0304-3959(95)00149-2
- Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther. (2012) 20:160-6. doi: 10.1179/2042618612Y.0000000001
- Cella D, Bullinger M, Scott C, Barofsky I, Aaronson N, Berzon R, et al. Group vs individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clin Proc. (2002) 77:384- 92. doi: 10.4065/77.4.384
- Falissard B, Sapin C, Loze JY, Landsberg W, Hansen K. Defining the minimal clinically important difference (MCID) of the Heinrichs-carpenter quality of life scale (QLS). Int J Methods Psychiatr Res. (2016) 25:101- 11. doi: 10.1002/mpr.1483
- Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimer's Dement Transl Res Clin Interv. (2019) 5:354-63. doi: 10.1016/j.trci.2019. 06.005
- Amri I, Millier A, Toumi M. Minimum clinically important difference in the global assessment functioning. Value Health. (2014) 17:A765- 6. doi: 10.1016/j.jval.2014.08.285
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J Consult Clin Psychol. (1999) 67:300- 7. doi: 10.1037/0022-006X.67.3.300
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. (1991) 59:12-9. doi: 10.1037/0022-006X.59.1.12
- de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes. (2006) 4:54. doi: 10.1186/1477-7525-4-54
- Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. (1999) 37:469- 78. doi: 10.1097/00005650-199905000-00006
- Muller K, Cohen J. Statistical power analysis for the behavioral sciences. Technometrics. (1989) 31:499-500. doi: 10.1080/00401706.1989.10488618
- Suzuki T. Which rating scales are regarded as "the standard" in clinical trials for schizophrenia? a critical review. Psychopharmacol Bull. (2011) 44:18-31.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. (2005) 79:231-8. doi: 10.1016/j.schres.2005.04.008
- Hermes EDA, Sokoloff D, Stroup TS, Rosenheck RA. Minimum clinically important difference in the positive and negative syndrome scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry. (2012) 73:526-32. doi: 10.4088/JCP.11m07162
- Marder SR, Daniel DG, Alphs L, Awad AG, Keefe RSE. Methodological issues in negative symptom trials. Schizophr Bull. (2011) 37:250-4. doi: 10.1093/schbul/sbq161
- Leucht S, Barabássy Á, Laszlovszky I, Szatmári B, Acsai K, Szalai E, et al. Linking PANSS negative symptom scores with the Clinical Global Impressions Scale: understanding negative symptom scores in schizophrenia. Neuropsychopharmacology. (2019) 44:1589-96. doi: 10.1038/s41386-019-0363-2
- Rabinowitz J, Werbeloff N, Caers I, Mandel FS, Stauffer V, Menard F, et al. Negative symptoms in schizophrenia -the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. (2013) 150:334-8. doi: 10.1016/j.schres.2013.06.023
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519-34. doi: 10.2147/NDT.S225643
- Harvey PD, Bellack AS. Toward a terminology for functional recovery in schizophrenia: is functional remission a viable concept? Schizophr Bull. (2009) 35:300-6. doi: 10.1093/schbul/sbn171
- Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. (2012) 11:73- 9. doi: 10.1016/j.wpsyc.2012.05.004
- Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context- related factors on real-life functioning of people with schizophrenia. World Psychiatry. (2014) 13:275-87. doi: 10.1002/wps.20167
- Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. (2017) 389:1103-13. doi: 10.1016/S0140-6736(17)30060-0
- Redelmeier DA, Lorig K. Assessing the clinical importance of symptomatic improvements: an illustration in rheumatology. Arch Intern Med. (1993) 153:1337-42. doi: 10.1001/archinte.1993.004101100 45008
- Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J Clin Epidemiol. (2017) 82:128-36. doi: 10.1016/j.jclinepi.2016.11.016
- Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology. (2005) 16:73-81. doi: 10.1097/01.ede.0000147512.81
- Powers DMW. Evaluation: From Precision, Recall and F-Factor. Tech Rep SEI-07-001. Adelaide, SA: Flinders University of South Australia (2007).
- Bland JM, Altman DG. Regression towards the mean. BMJ. (1994) 308:1499. doi: 10.1136/bmj.308.6942.1499
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. (2007) 4:28-37.
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. (2015) 41:892- 9. doi: 10.1093/schbul/sbu170
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr Res. (2017) 186:55-62. doi: 10.1016/j.schres.2016. 05.015
- Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. (2011) 34:3-78. doi: 10.1177/0269881119889296
![FIGURE 2 | Receiver Operating Characteristic (ROC) curve: predictive accuracy of the PANSS-FSNS (Positive and Negative Syndrome Scale Factor Score for Negative Symptoms) scale for differentiating minimally improved vs. clinically unchanged status. The values on the vertical and horizontal axis, respectively, depict the sensitivity (“true positive rate”) and 1— specificity (“false positive rate”) values for the differentiation as a function of change from baseline in the PANSS-FSNS scale. The leftmost part of the ROC curve represents the highest empirically observed improvements in the sample as compared to baseline while the rightmost part represents no improvement (or even deterioration). Please note that the ROC curve for differentiating the minimally improved from the clinically unchanged status based on the PANSS-FSNS (ROC model, depicted in blue in the figure) significantly outperforms the random classification (ROC1 model, in red), with an area under the curve (AUG, labeled as “Area”) value of 0.7232 vs. 0.5000 (0 < 0.0001). PANSS-FSNS, Positive and Negative Syndrome Scale-Factor Score for Negative Symptoms; n, number of events; CGI-I, Clinical Global Impression—Improvement; MCID, and MCIDo, Minimum clinically important differences according to the definitions in the text; SD, Standard deviation; LSMD, Least-square mean difference between [1] and [2], as estimated by MMR\M, with corresponding 95% confidence limits. Please also note that for the computation of % changes from baseline the value of 7 was subtracted from the observed baseline severity (i.e., 27.6) since the minimum value of the PANSS-FSNS factor is 7 (i.e., the symptoms on all seven constituting items of the factor are rated as “Absent’).](https://figures.academia-assets.com/114064560/figure_002.jpg) ](
](