Efficient RAFT polymerization of N-(3-aminopropyl)methacrylamide hydrochloride using unprotected “clickable” chain transfer agents (original) (raw)
Abstract
The reversible addition fragmentation chain transfer (RAFT) of N-(3-aminopropyl)methacrylamide hydrochloride (APMA) using unprotected ''clickable'' chain transfer agents in water/dioxane mixtures is reported. The controlled character of the polymerization was confirmed by the linear increase of the polymer molecular weight with monomer conversion, the narrow molecular weight distribution (Ð 6 1.1) and by chain extension experiments. The alkyne-terminated PAPMA was further functionalized by ''click'' chemistry with an azido-functionalized coumarin derivative. The method reported here will be useful for the preparation of novel PAPMA based materials for biomedical applications using a strategy that does not require challenging protection/deprotection steps.
Figures (8)
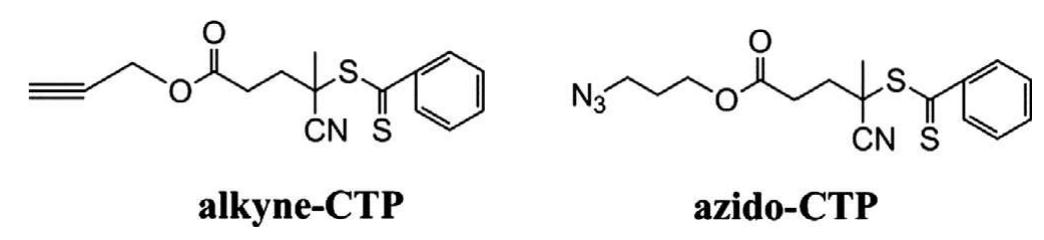
Fig. 1. Chemical structures of both alkyne-CTP and azido-CTP.
Kinetic parameters for the RAFT polymerization of APMA using azido-CTP or alkyne-CTP in water: 1,4-dioxane = 2:1 (v:v) at 70 °C. Conditions: [APMA]o = 1.87 M; [ACVA]o = 0 (molar ratio in comparison to the CTA number of moles).
Fig. 2. (a) Kinetic plots of In[M]o/[M] vs. time and (b) plot of number-average molecular weight (M°"*) and D (My/M,) vs. monomer conversion (the dashed line represents the theoretical molecular weight at a given conversion) for the RAFT of APMA at 70 °C in a water:1,4-dioxane = 2:1 (v:v) mixture using CTP (red symbols) and alkyne-CTP (black symbols) as chain transfer agents (CTA). Reaction conditions: [APMA]o/[CTA]o/[ACVA]o = 100/1/0.5; [APMA]po = 1.87 M. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) * An ACVA molar ratio of 0.2 was used in this polymerization.
Fig. 3. (a) Kinetic plots of In[M]o/[M] vs. time and (b) plot of the number-average molecular weight (M;"°) and D (Mw/Mn) vs. monomer conversion (the dashed lin represents the theoretical molecular weight at a given conversion) for the RAFT of APMA at 70°C in water:1,4-dioxane = 2:1 (v:v) (black symbols) and water:1,4 dioxane = 1:1 (v:v) (red symbols) mixtures. Reaction conditions: [APMA]9/[azido-CTP]o/[ACVA]o = 100/1/0.5; [APMA]) = 1.87 M. (For interpretation of the references to colo in this figure legend, the reader is referred to the web version of this article.) Nevertheless, the kinetic results showed that the azido-CTP did not allow the same level of control over the PAPMA molecular Alternatively, to use the alkyne functionality, one can take advantage of azide terminated polymers for click chemistry pur- poses. An experimental study reported by Ladmiral and co-workers
‘ig. 4. SEC chromatograms of the PAPMA samples during the RAFT polymerization at 70 °C in (a) a water:1,4-dioxane = 2:1 (v:v) mixture and (b) a water:1,4-dioxane = 1: v:v) mixture. Conditions: [APMA]o/[azido-CTP]o/[ACVA]o = 100/1/0.5; [APMA]o = 1.87 M. weight as did both CTP and alkyne-CTP for the same experimental conditions (black symbols in Fig. 3). The D of the polymers was slightly higher, primarily due to the presence of tailing in the molecular weight distribution curves (Fig. 4(a)). This observation could be due to radical termination reactions that occurred in the beginning of the polymerization. By comparing the area of the low molecular weight shoulder with the total area of the SEC chro- matogram, it was possible to estimate that after 30 min of poly- merization, there were ~27% dead polymer chains. We anticipated that the poor control exhibited by the azido-CTP could be associated with the low solubility of this compound in the reac- tion solvent mixture, even at 70 °C. In comparison with both CTP and alkyne-CTP, the azido-CTP R group has a longer aliphatic chain, which decreases its solubility in water. To confirm this hypothesis, the effect of the solvent mixture composition on the control of the RAFT polymerization of PAPMA mediated by the azido-CTP was also examined. The percentage of dioxane in the solvent mixture was increased up to 50%, and the other experimental conditions were conserved. The results showed that significant improvements were achieved in terms of polymerization control, with a 64% decrease in the PAPMA dead chains (Fig. 4(b)) as well as a 39% increase in the maximum monomer conversion (red symbols in Fig. 3). In addition, better control was achieved, as evidenced by the lower D of the PAPMA and the agreement between the exper- imental molecular weights and the theoretical ones (dashed line in Fig. 3(b)). These results corroborate the idea that the poorer control exhibited by the azido-CTP in this particular RAFT system was related to its solubility in the reaction solvent mixture. Some authors have suggested that the marked chain transfer activity dif- ferences between clickable CTAs derived from the same molecule
Fig. 5. 400 MHz 'H NMR spectra in D0 of (a) the alkyne-terminated PAPMA obtained by RAFT polymerization using [APMA]o/[alkyne-CTP]o/[ACVA]o = 25/1/0.2 (molar ratios) in water: 1,4-dioxane = 2:1 (v:v) at 70 °C for 5 h. Conv. = 82%; Me =3.7 x 10’; M=°10.4 x 10?; D = 1.19; (b) azide-terminated PAPMA obtained by RAFT polymerization using [APMA])/[azido-CTP]o/[ACVA]o = 25/1/0.5 (molar ratios) in water:1,4-dioxane = 2:1 (v:v) at 70 °C for 4h. Conv. = 94%; M™ = 4.5 x 107; MS = 16.3 x 10°; D=1.21.
Fig. 6. SEC traces of the macro-PAPMA (alkyne-terminated) and extended PAPMA obtained by RAFT polymerization at 70 °C in water:1,4-dioxane = 2:1 (v:v).
Fig. 7. 400 MHz 'H NMR spectrum in D,0 of the purified coumarin-functionalized PAPMA obtained by a click reaction One of the most effective ways to demonstrate the “livingness” of polymers is to perform a chain extension reaction, in which the We reported the synthesis of clickable poly(N-(3-aminopro- pyl)methacrylamide) (PAPMA) by RAFT polymerization using azido-CTP or alkyne-CTP as chain transfer agents without any pro- tection/deprotection steps. Both clickable CTAs exhibited similar or higher chain transfer activity compared to the commercial ana- logue CTP under the experimental conditions used. The control over the PAPMA molecular weight was very good, as the polymer

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (47)
- W.A. Braunecker, K. Matyjaszewski, Prog. Polym. Sci. 32 (2007) 93-146.
- G. Moad, Aust. J. Chem. 59 (2006) 661-662.
- S. Perrier, P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem. 43 (2005) 5347- 5393.
- W. Zhang, F. D'Agosto, P.-Y. Dugas, J. Rieger, B. Charleux, Polymer 54 (2013) 2011-2019.
- C. Lin, H. Zhan, M. Liu, Y. Habibi, S. Fu, L.A. Lucia, J. Appl. Polym. Sci. 127 (2013) 4840-4849.
- D.B. Thomas, B.S. Sumerlin, A.B. Lowe, C.L. McCormick, Macromolecules 36 (2003) 1436-1439.
- A.J. Convertine, N. Ayres, C.W. Scales, A.B. Lowe, C.L. McCormick, Biomacromolecules 5 (2004) 1177-1180.
- D.B. Thomas, A.J. Convertine, L.J. Myrick, C.W. Scales, A.E. Smith, A.B. Lowe, Y.A. Vasilieva, N. Ayres, C.L. McCormick, Macromolecules 37 (2004) 8941-8950.
- X. Liu, X. Feng, J. Chen, Y. Cao, J. Macrom, Sci. Part A: Pure Appl. Chem. 50 (2012) 65-71.
- F. Huo, X. Wang, Y. Zhang, X. Zhang, J. Xu, W. Zhang, Macromol. Chem. Phys. 214 (2013) 902-911.
- S. Xu, J. Huang, S. Xu, Y. Luo, Polymer 54 (2013) 1779-1785.
- G. Moad, Y.K. Chong, A. Postma, E. Rizzardo, S.H. Thang, Polymer 46 (2005) 8458-8468.
- M.A. Harvison, P.J. Roth, T.P. Davis, A.B. Lowe, Aust. J. Chem. 64 (2011) 992- 1006.
- P.J. Roth, C. Boyer, A.B. Lowe, T.P. Davis, Macromol. Rapid Commun. 32 (2011) 1123-1143.
- H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 40 (2001) 2004- 2021.
- J.E. Moses, A.D. Moorhouse, Chem. Soc. Rev. 36 (2007) 1249-1262.
- A. Gregory, M.H. Stenzel, Prog. Polym. Sci. 37 (2012) 38-105.
- A. Hasneen, H.S. Han, H.-J. Paik, React. Funct. Polym. 69 (2009) 681-687.
- D. Fournier, R. Hoogenboom, U.S. Schubert, Chem. Soc. Rev. 36 (2007) 1369- 1380.
- M.R. Whittaker, C.N. Urbani, M.J. Monteiro, J. Am. Chem. Soc. 128 (2006) 11360-11361.
- V. Ladmiral, G. Mantovani, G.J. Clarkson, S. Cauet, J.L. Irwin, D.M. Haddleton, J. Am. Chem. Soc. 128 (2006) 4823-4830.
- J.A. Opsteen, J.C.M. van Hest, Chem. Commun. (2005) 57-59.
- P.L. Golas, K. Matyjaszewski, Chem. Soc. Rev. 39 (2010) 1338-1354.
- W. Yuan, J. Zhang, H. Zou, J. Ren, J. Polym. Sci., Part A: Polym. Chem. 50 (2012) 2541-2552.
- M. Semsarilar, V. Ladmiral, S. Perrier, Macromolecules 43 (2010) 1438-1443.
- J. Yang, K. Luo, H. Pan, P. Kopec ˇková, J. Kopec ˇek, React. Funct. Polym. 71 (2011) 294-302.
- P.D. Topham, N. Sandon, E.S. Read, J. Madsen, A.J. Ryan, S.P. Armes, Macromolecules 41 (2008) 9542-9547.
- Y.T. Li, S.P. Armes, Macromolecules 42 (2009) 939-945.
- E.S. Read, K.L. Thompson, S.P. Armes, Polym. Chem. 1 (2010) 221-230.
- A.W. York, F. Huang, C.L. McCormick, Biomacromolecules 11 (2010) 505-514.
- A.W. York, Y. Zhang, A.C. Holley, Y. Guo, F. Huang, C.L. McCormick, Biomacromolecules 10 (2009) 936-943.
- X. Xu, J.D. Flores, C.L. McCormick, Macromolecules 44 (2011) 1327-1334.
- X. Xu, A.E. Smith, S.E. Kirkland, C.L. McCormick, Macromolecules 41 (2008) 8429-8435.
- X. Xu, A.E. Smith, C.L. McCormick, Aust. J. Chem. 62 (2009) 1520-1527.
- Z. Deng, H. Bouchékif, K. Babooram, A. Housni, N. Choytun, R. Narain, J. Polym. Sci., Part A: Polym. Chem. 46 (2008) 4984-4996.
- Y. Li, B.S. Lokitz, C.L. McCormick, Angew. Chem. 118 (2006) 5924-5927.
- G. Gao, K. Yu, J. Kindrachuk, D.E. Brooks, R.E.W. Hancock, J.N. Kizhakkedathu, Biomacromolecules 12 (2011) 3715-3727.
- A.H. Alidedeoglu, A.W. York, D.A. Rosado, C.L. McCormick, S.E. Morgan, J. Polym. Sci., Part A: Polym. Chem. 48 (2010) 3052-3061.
- B.J. Adzima, C.N. Bowman, AlChE J. 58 (2012) 2952-2965.
- K. Sivakumar, F. Xie, B.M. Cash, S. Long, H.N. Barnhill, Q. Wang, Org. Lett. 6 (2004) 4603-4606.
- D. Quemener, T.P. Davis, C. Barner-Kowollik, M.H. Stenzel, Chem. Commun. (2006) 5051-5053.
- Y.A. Vasilieva, C.W. Scales, D.B. Thomas, R.G. Ezell, A.B. Lowe, N. Ayres, C.L. McCormick, J. Polym. Sci., Part A: Polym. Chem. 43 (2005) 3141-3152.
- D.B. Thomas, A.J. Convertine, R.D. Hester, A.B. Lowe, C.L. McCormick, Macromolecules 37 (2004) 1735-1741.
- V. Ladmiral, T.M. Legge, Y. Zhao, Macromolecules 41 (2008) 6728-6732.
- V.K. Patel, N.K. Vishwakarma, A.K. Mishra, C.S. Biswas, P. Maiti, B. Ray, J. Appl. Polym. Sci. 127 (2013) 4305-4317.
- J. Lu, W. Zhang, S.-J. Richards, M.I. Gibson, G. Chen, Polym. Chem. 5 (2014) 2326-2332.
- S.R. Trenor, A.R. Shultz, B.J. Love, T.E. Long, Chem. Rev. 104 (2004) 3059-3078.
![Kinetic parameters for the RAFT polymerization of APMA using azido-CTP or alkyne-CTP in water: 1,4-dioxane = 2:1 (v:v) at 70 °C. Conditions: [APMA]o = 1.87 M; [ACVA]o = 0 (molar ratio in comparison to the CTA number of moles).](https://figures.academia-assets.com/44278929/table_001.jpg) ](
](![Fig. 2. (a) Kinetic plots of In[M]o/[M] vs. time and (b) plot of number-average molecular weight (M°"*) and D (My/M,) vs. monomer conversion (the dashed line represents the theoretical molecular weight at a given conversion) for the RAFT of APMA at 70 °C in a water:1,4-dioxane = 2:1 (v:v) mixture using CTP (red symbols) and alkyne-CTP (black symbols) as chain transfer agents (CTA). Reaction conditions: [APMA]o/[CTA]o/[ACVA]o = 100/1/0.5; [APMA]po = 1.87 M. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) * An ACVA molar ratio of 0.2 was used in this polymerization.](https://figures.academia-assets.com/44278929/figure_002.jpg) ](
](![Fig. 3. (a) Kinetic plots of In[M]o/[M] vs. time and (b) plot of the number-average molecular weight (M;"°) and D (Mw/Mn) vs. monomer conversion (the dashed lin represents the theoretical molecular weight at a given conversion) for the RAFT of APMA at 70°C in water:1,4-dioxane = 2:1 (v:v) (black symbols) and water:1,4 dioxane = 1:1 (v:v) (red symbols) mixtures. Reaction conditions: [APMA]9/[azido-CTP]o/[ACVA]o = 100/1/0.5; [APMA]) = 1.87 M. (For interpretation of the references to colo in this figure legend, the reader is referred to the web version of this article.) Nevertheless, the kinetic results showed that the azido-CTP did not allow the same level of control over the PAPMA molecular Alternatively, to use the alkyne functionality, one can take advantage of azide terminated polymers for click chemistry pur- poses. An experimental study reported by Ladmiral and co-workers](https://figures.academia-assets.com/44278929/figure_003.jpg) ](
](![‘ig. 4. SEC chromatograms of the PAPMA samples during the RAFT polymerization at 70 °C in (a) a water:1,4-dioxane = 2:1 (v:v) mixture and (b) a water:1,4-dioxane = 1: v:v) mixture. Conditions: [APMA]o/[azido-CTP]o/[ACVA]o = 100/1/0.5; [APMA]o = 1.87 M. weight as did both CTP and alkyne-CTP for the same experimental conditions (black symbols in Fig. 3). The D of the polymers was slightly higher, primarily due to the presence of tailing in the molecular weight distribution curves (Fig. 4(a)). This observation could be due to radical termination reactions that occurred in the beginning of the polymerization. By comparing the area of the low molecular weight shoulder with the total area of the SEC chro- matogram, it was possible to estimate that after 30 min of poly- merization, there were ~27% dead polymer chains. We anticipated that the poor control exhibited by the azido-CTP could be associated with the low solubility of this compound in the reac- tion solvent mixture, even at 70 °C. In comparison with both CTP and alkyne-CTP, the azido-CTP R group has a longer aliphatic chain, which decreases its solubility in water. To confirm this hypothesis, the effect of the solvent mixture composition on the control of the RAFT polymerization of PAPMA mediated by the azido-CTP was also examined. The percentage of dioxane in the solvent mixture was increased up to 50%, and the other experimental conditions were conserved. The results showed that significant improvements were achieved in terms of polymerization control, with a 64% decrease in the PAPMA dead chains (Fig. 4(b)) as well as a 39% increase in the maximum monomer conversion (red symbols in Fig. 3). In addition, better control was achieved, as evidenced by the lower D of the PAPMA and the agreement between the exper- imental molecular weights and the theoretical ones (dashed line in Fig. 3(b)). These results corroborate the idea that the poorer control exhibited by the azido-CTP in this particular RAFT system was related to its solubility in the reaction solvent mixture. Some authors have suggested that the marked chain transfer activity dif- ferences between clickable CTAs derived from the same molecule](https://figures.academia-assets.com/44278929/figure_004.jpg) ](
](![Fig. 5. 400 MHz 'H NMR spectra in D0 of (a) the alkyne-terminated PAPMA obtained by RAFT polymerization using [APMA]o/[alkyne-CTP]o/[ACVA]o = 25/1/0.2 (molar ratios) in water: 1,4-dioxane = 2:1 (v:v) at 70 °C for 5 h. Conv. = 82%; Me =3.7 x 10’; M=°10.4 x 10?; D = 1.19; (b) azide-terminated PAPMA obtained by RAFT polymerization using [APMA])/[azido-CTP]o/[ACVA]o = 25/1/0.5 (molar ratios) in water:1,4-dioxane = 2:1 (v:v) at 70 °C for 4h. Conv. = 94%; M™ = 4.5 x 107; MS = 16.3 x 10°; D=1.21.](https://figures.academia-assets.com/44278929/figure_005.jpg) ](
](
