Effect of gellan, alone and in combination with high-methoxy pectin, on the structure and stability of doogh, a yogurt-based Iranian drink (original) (raw)
Abstract
Doogh is a traditional Iranian drink prepared by fragmentation and dilution of yogurt, with addition of salt and flavouring. In the present work, we have used viscometry, microscopy, particle-size analysis and measurements of serum separation to explore the effect of very low concentrations of gellan gum (0.01, 0.03 and 0.05 wt %), alone or in combination with 0.25 wt % high-methoxy pectin (HMP), on its structure and stability. HMP is known to prevent association of casein particles in acidic milk drinks by steric stabilisation, forming a protective layer bound electrostatically to the surface of the particles. Doogh incorporating 0.25 wt % HMP alone showed satisfactory stability on storage for w10 days at 5 C, but after 15 days there was obvious separation into a dense sediment and a much clearer upper layer that occupied more than 80% of the total volume, which we attribute to progressive sedimentation of individual sterically-stabilised particles. Samples incorporating gellan (with or without HMP), by contrast, showed rapid development of a clear serum phase, with little further separation at longer times (up to w1 month), suggesting expression of fluid by contraction of a gel network (i.e. syneresis rather than sedimentation). Particle size increased dramatically (more than 10-fold) with increasing concentration of gellan, and at the highest concentration studied (0.05 wt %) a continuous network of casein-rich strands was observed by phase-contrast microscopy. The concentration of NaCl used in the doogh samples (0.5 wt % z 85 mM) is known to be sufficient to maintain gellan in its ordered (double-helix) conformation, which has higher charge density than individual molecules of HMP. We suggest that network structure is formed by electrostatic attachment of gellan to fragments of acid-casein gel, thus increasing particle size and inhibiting surface-coverage by HMP, with weaker associations between gellan helices allowing the samples to flow. Observed decrease in serum volume with increasing concentration of gellan is attributed to formation of progressively stronger coupled networks with greater resistance to syneresis. Stabilisation of doogh with 0.05 wt % gellan in combination with 0.25 wt % HMP had no adverse effect on organoleptic acceptability, and reduced serum volume on protracted storage to w10% (in comparison with over 80% for the same concentration of HMP alone), suggesting that gellan could be of practical value in extending the shelf-life of doogh (and related acidic milk drinks).
Figures (14)
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Provided for non-commercial research and education use. Not for reproduction, distribution or commercial use.
Parameters derived from double-logarithmic plots of specific viscosity (1sp) versus shear rate (7): slope; intercept at 7 = 1 s~!; correlation coefficient (17).
Fig. 1. Flow curves (20 °C) of shear stress versus shear rate for illustrative samples of doogh (Batch 3) incorporating (a) 0.03 wt % gellan or (b) 0.03 wt % gellan plus 0.25 wt % HMP, after the polysaccharides had been dissolved at temperatures of 70(©), 80 (A) or 90 (C1) °C.
Fig. 2. Shear-rate dependence of (a) viscosity and (b) specific viscosity for doogh (Batch 3) alone (©), and with gellan at concentrations (wt %) of 0.01 ( @), 0.03 (A) anc 0.05 (a).
Fig. 3. Variation of viscosity (1 s~!; 20°C) with gellan concentration in preparations (a) with no added pectin and (b) with 0.25 wt % HMP, for polymer solutions alone (O) and in combination with doogh from Batches 1 (a), 2 (@) and 3 (A).
Fig. 4. Comparison of viscosity (1 s~!; 20°C) for doogh samples 1 (a), 2 (@) and 3 (A) with (vertical axis) and without (horizontal axis) 0.25 wt % HMP. The dashed line corresponds to equal viscosities in the presence and absence of pectin; the horizontal solid line shows the viscosity of 0.25 wt % HMP alone. Fig. 4 shows the viscosities of the three batches of plain doogh ‘with no added polysaccharides) in comparison with the corre- sponding values for preparations incorporating 0.25 wt % HMP (but no gellan), and with the viscosity of 0.25 wt % HMP alone. Incor- poration of pectin in the weakest batch of doogh (Batch 2) raised the viscosity to a value only slightly above that of HMP alone. For the strongest batch (Batch 1), the viscosity of the preparation with added pectin was virtually identical to that of the corresponding sample of plain doogh (with no pectin). Incorporation of HMP in the intermediate batch (Batch 3) caused only a slight increase in viscosity. The overall viscosity therefore seems to be dominated by pectin when the doogh to which it is added is substantially less viscous than the pectin alone, with the doogh becoming the dominant component when it is substantially more viscous than the pectin.
Fig. 6. Microstructure of “plain doogh”, with no added gellan or pectin (top left-hand image in Fig. 5), at higher magnification (scale bar denotes 60 1m). On incorporation of gellan (0.01, 0.03 and 0.05 wt %), with or without HMP, the distribution becomes less homogeneous, and at the highest concentration of gellan (0.05 wt %) has the appearance of a continuous crosslinked network, with the thickness of some strands approaching ~0.3mm. Even larger assemblies (with dimensions up to ~1 mm) can be seen at the lower concentrations of gellan (0.01 and 0.03 wt %), but these do not appear to form a continuous network, and are less evident in the samples that also include 0.25 wt % HMP.
Fig. 5. Microstructure of doogh samples (Batch 2) visualised by phase-contrast microscopy. Proteins are stained by Rhodamine B and appear as bright zones in the micrographs. Magnification is the same in all images (scale bars denote 250 1m). Top row: samples with no added pectin; bottom row: samples incorporating 0.25 wt % HMP; gellan concentrations (from left to right within each row) of 0, 0.01, 0.03 and 0.05 wt %.
Fig. 7. Particle-size distribution for doogh samples (Batch 2) prepared (a) without pectin and (b) with 0.25 wt % HMP at gellan concentrations (wt %) of 0 (©), 0.01 (@), 0.03 (A) and 0.05 (a). As shown in Fig. 7, the particles detected in plain doogh by the Malvern Mastersizer have a broad size-distribution, extending to ~25 um (comparable to the dimensions of the particles in the micrograph shown as Fig. 6) and centred at ~6—7 um. A peak at
Fig. 8. Variation of mean particle diameter with gellan concentration for doogh with no added pectin (©) and with 0.25 wt % HMP (@). Four preparations of doogh from Batch 2 were assessed: plain doogh, and samples incorporating 0.05 wt % gellan and/or 0.25 wt % HMP. The samples were rated for aroma, taste, homogeneity, mouthfeel and overall acceptability, using a 5-point hedonic scale.
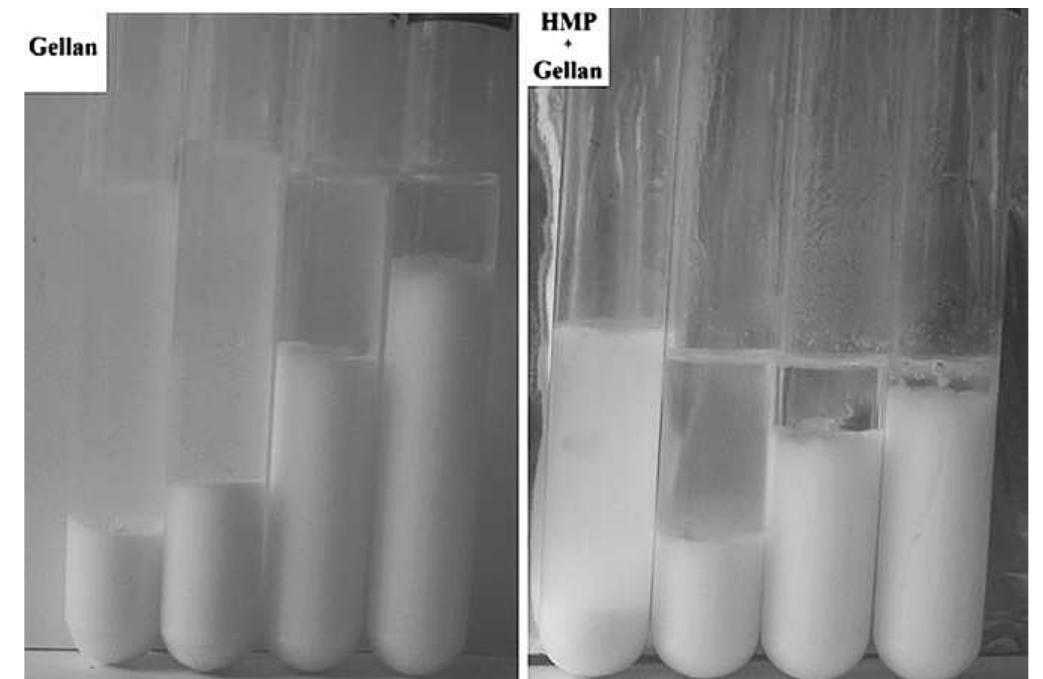
Fig. 9. Separation of serum from doogh samples (Batch 2) after storage for 15 days at 5 °C, without pectin (left-hand frame) and with 0.25 wt % HMP (right-hand frame). The concentrations of gellan used (from left to right within each frame) were 0, 0.01, 0.03 and 0.05 wt %.
Fig. 10. Separation of serum layer (Fig. 9) from doogh (Batch 2) on storage at 5 °C, alone (*) and in combination with gellan at concentrations (wt %) of 0.01 (circles), 0.03 (triangles) or 0.05 (squares) in the presence (filled symbols) or absence (open symbols) of 0.25 wt % HMP. The time-course of separation is shown in Fig. 10. Development of an upper serum layer and a dense, opaque lower layer occurs
Fig. 11. Correlation between viscosity and amount of serum formed after storage for 1 day at 5 °C (Fig. 10) for doogh samples prepared with (@) and without (©) 0.25 wt % HMP at gellan concentrations of 0, 0.01, 0.03 and 0.05 wt %. Ss Ne ore eee eee Js eee eee OE Development of a serum layer by samples incorporating gellan (or by plain doogh), by contrast, resembled expression of fluid from a contracting gel network (i.e. syneresis rather than sedimentation).
Fig. 12. Correlation between viscosity and amount of serum formed after storage for (a) 5 days and (b) 15 days at 5 °C (Fig. 10) for doogh samples prepared with (@) and without (©) 0.25 wt % HMP at gellan concentrations of 0, 0.01, 0.03 and 0.05 wt %.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (72)
- Arnott, S., Scott, W. E., Rees, D. A., & McNab, C. G. A. (1974). i-Carrageenan: molecular structure and packing of polysaccharide double helices in oriented fibres of divalent cation salts. Journal of Molecular Biology, 90, 253e267.
- Antonov, Y. A., Grinberg, V. Y., Zhuravskya, N. A., & Tolstoguzov, V. B. (1982). Concentration of the proteins of skimmed milk by membraneless, isobaric osmosis. Carbohydrate Polymers, 2, 81e90.
- Arshad, M., Paulsson, M., & Dejmek, P. (1993). Rheology of buildup, breakdown and rebodying of acid casein gels. Journal of Dairy Science, 76, 3310e3316.
- Bohdanecký, M., & Kovár, J. (1982). Viscosity of polymer solutions. Amsterdam: Elsevier.
- Bourriot, S., Garnier, C., & Doublier, J. L. (1999). Phase separation, rheology and micro- structure of micellar caseineguar gum mixtures. Food Hydrocolloids, 13, 43e49.
- Chandrasekaran, R., & Thailambal, V. G. (1990). The influence of calcium ions, acetate and L-glycerate groups on the gellan double-helix. Carbohydrate Poly- mers, 12, 431e442.
- Chandrasekaran, R., Millane, R. P., Arnott, S., & Atkins, E. D. T. (1988). The crystal structure of gellan. Carbohydrate Research, 175, 1e15.
- Christensen, S. H. (1986). Pectins. In M. Glicksman (Ed.), Food hydrocolloids, Vol. III (pp. 205e230). Boca Raton, Florida, USA: CRC Press.
- Cobos, A., Horne, D. S., & Muir, D. D. (1995). Rheological properties of acid milk gels. I. Effect of composition, process and acidification conditions on products from recombined milks. Milchwissenschaft, 50, 444e448.
- Crescenzi, V., & Dentini, M. (1988). Solution conformation of the polysaccharide gellan. In G. O. Phillips, D. J. Wedlock, & P. A. Williams (Eds.), Gums and sta- bilisers for the food industry, 4 (pp. 63e69). Oxford, UK: IRL Press.
- Crescenzi, V., Dentini, M., Coviello, T., & Rizzo, R. (1986). Comparative analysis of the behaviour of gellan gum (S-60) and welan gum (S-130) in dilute aqueous solution. Carbohydrate Research, 149, 425e432.
- Dickinson, E. (1992). An introduction to food colloids. UK: Oxford University Press.
- Dickinson, E. (2003). Hydrocolloids at interfaces and the influence on properties of dispersed systems. Food Hydrocolloids, 17, 25e39.
- Dickinson, E., Semenova, M. G., Antipova, A. S., & Pelan, E. G. (1998). Effect of high- methoxy pectin on properties of casein-stabilized emulsions. Food Hydrocol- loids, 12, 425e432.
- Doesburg, J. J., & de Vos, L. (1959). Pasteurized mixtures of fruit jus and milk, with long shelf life. International Fruit Jus Congress 32e37.
- Fox, P. F., & McSweeney, P. L. H. (1998). Dairy chemistry and biochemistry. London: Blackie Academic & Professional.
- Glahn, P. E. (1982). Hydrocolloid stabilisation of protein suspensions at low pH. Progress in Food and Nutrition Science, 6, 171e177.
- Glahn, P. E., & Rolin, C. (1996). Properties and food uses of pectin fractions. In G. O. Phillips, D. J. Wedlock, & P. A. Williams (Eds.), Gums and stabilisers for the food industry 8 (pp. 393e402). Oxford, UK: Pergamon Press.
- Grant, G. T., Morris, E. R., Rees, D. A., Smith, P. J. C., & Thom, D. (1973). Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Letters, 32, 195e198.
- Grasdalen, H., & Smidsrød, O. (1987). Gelation of gellan gum. Carbohydrate Polymers, 7, 371e393.
- Grinberg, V. Y., & Tolstoguzov, V. B. (1997). Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocolloids, 11, 145e158.
- Hemar, Y., Tamehana, M., Munro, P. A., & Singh, H. (2001). Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocolloids, 15, 565e574.
- Holt, C. (1992). Structure and stability of bovine casein micelles. Advances in Protein Chemistry, 43, 63e151.
- Holt, C., & Horne, D. S. (1996). The hairy casein micelle: evolution of the concept and its implications for dairy technology. Netherlands Milk and Dairy Journal, 50, 85e111.
- Horne, D. S., & Davidson, C. M. (1993). Influence of heat treatment on gel formation in acidified milks. In Protein and fat globule modification by heat treatment, homogenisation and other technological means for high quality dairy products (pp 267e276). Brussels: International Dairy Federation (IDF Special Issue 9303).
- Huang, X., Kakuda, Y., & Cui, W. (2001). Hydrocolloids in emulsions: particle size distribution and interfacial activity. Food Hydrocolloids, 15, 533e542.
- Jansson, P.-E., Lindberg, B., & Sandford, P. A. (1983). Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohy- drate Research, 124, 135e139.
- Kiani, H., Ebrahimzadeh-Mousavi, M.-A., Emam-Djomeh, Z., & Yarmand, M. S. (2008). Effect of concentration and hydration temperature of gellan gum on the stability and physical properties of acidified milk protein solutions. Australian Journal of Dairy Technology, 63, 94e99.
- Kiani, H., Mousavi, S. M., & Emam-Djomeh, Z. (2008). Rheological properties of Iranian yoghurt drink, doogh. International Journal of Dairy Science, 3, 71e78.
- Köksoy, A., & Kiliç, M. (2003). Effects of water and salt level on rheological properties of ayran, a Turkish yoghurt drink. International Dairy Journal, 13, 835e839.
- Koksoy, A., & Kilic, M. (2004). Use of hydrocolloids in textural stabilization of a yoghurt drink, ayran. Food Hydrocolloids, 18, 593e600, 13.
- Kravtchenko, T. P., Parker, A., & Trespoey, A. (1995). Colloidal stability and sedi- mentation of pectin-stabilized acid milk drinks. In E. Dickinson, & D. Lorient (Eds.), Food macromolecules and colloids (pp. 349e355). Cambridge, UK: Royal Society of Chemistry.
- Kuo, M.-S., Mort, A. J., & Dell, A. (1986). Identification and location of L-glycerate, an unusual acyl substituent in gellan gum. Carbohydrate Research, 156, 173e187.
- Langendorff, V., Cuvelier, G., Launay, B., & Parker, A. (1997). Gelation and floccula- tion of casein micelle/carrageenan mixtures. Food Hydrocolloids, 11, 35e40.
- Laurent, M. A., & Boulenguer, P. (2003). Stabilization mechanism of acid dairy drinks (ADD) induced by pectin. Food Hydrocolloids, 17, 445e454.
- Lucey, J. A., van Vliet, T., Grolle, K., Geurts, T., & Walstra, P. (1997). Properties of acid casein gels made by acidification with glucono-d-lactone. 1. Rheological prop- erties. International Dairy Journal, 7, 381e388.
- Lucey, J. A., Tamenaha, M., Singh, H., & Munro, P. A. (1999). Stability of model acid milk beverage: effect of pectin concentration, storage temperature and milk heat treatment. Journal of Texture Studies, 30, 305e318.
- Maroziene, A., & de Kruif, C. G. (2000). Interaction of pectin and casein micelles. Food Hydrocolloids, 14, 391e394.
- Matsukawa, S., & Watanabe, T. (2007). Gelation mechanism and network structure of mixed solution of low-and high-acyl gellan studied by dynamic viscoelas- ticity, CD and NMR measurements. Food Hydrocolloids, 21, 1355e1361.
- May, C. D. (1990). Industrial pectins: sources, production and applications. Carbo- hydrate Polymers, 12, 79e99.
- Milas, M., Shi, X., & Rinaudo, M. (1990). On the physicochemical properties of gellan gum. Biopolymers, 30, 451e464.
- Morawetz, H. (1965). Macromolecules in solution. New York: Wiley.
- Morris, E. R. (1990). Mixed polymer gels. In P. Harris (Ed.), Food gels (pp. 291e359). London: Elsevier.
- Morris, E. R., Gothard, M. G. E., Hember, M. W. N., Manning, C. E., & Robinson, G. (1996). Conformational and rheological transitions of welan, rhamsan and acylated gellan. Carbohydrate Polymers, 30, 163e175.
- Morris, E. R., Richardson, R. K., & Whittaker, L. E. (1999). Rheology and gelation of deacylated gellan polysaccharide with Na þ as the sole counterion. Progress in Colloid and Polymer Science, 114, 109e115.
- Mulvihill, D. M., & Grufferty, M. B. (1995). Effect of thermal processing on the coag- ulability of milk by acid. In P. F. Fox (Ed.), Heat induced changes in milk (2nd ed.). (pp. 188e205) Brussels: International Dairy Federation (IDF Special Issue 9501).
- Nakamura, A., Yoshida, R., Maedab, H., & Corrediga, M. (2006). The stabilizing behaviour of soybean soluble polysaccharide and pectin in acidified milk beverages. International Dairy Journal, 16, 361e369.
- O'Neill, M. A., Selvendran, R. R., & Morris, V. J. (1983). Structure of the acidic extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbo- hydrate Research, 124, 123e133.
- Paraskevopoulou, A., Athanasiadis, I., Blekas, G., Koutinas, A. A., Kanellaki, M., & Kiosseoglou, V. (2003). Influence of polysaccharide addition on stability of a cheese whey kefiremilk mixture. Food Hydrocolloids, 17, 615e620.
- Parker, A., Boulenguer, P., & Kravtchenko, T. P. (1994). Effect of addition of high methoxy pectin on the rheology and colloidal stability of acid milk drinks. In K. Nishiari, & E. Doi (Eds.), Food hydrocolloids: Structures, properties, and func- tions (pp. 307e312). New York: Plenum Press.
- Picullel, L., Bergfeldt, K., & Nilsson, S. (1995). Factors determining phase behaviour of multi component biopolymer systems. In S. E. Harding, S. E. Hill, & J. R. Mitchell (Eds.), Biopolymer mixtures (pp. 13e35). Nottingham, UK: Not- tingham University Press.
- Piculell, L., Nilsson, S., Falck, L., & Tjerneld, F. (1991). Phase separation in aqueous mixtures of similarly charged polyelectrolytes. Polymer Communications, 32, 158e160.
- Ridley, B. L., O'Neill, M. A., & Mohnen, D. (2001). Pectins: structure, biosynthesis, and oligogalacturonide-related signalling. Phytochemistry, 57, 929e967.
- Robinson, G., Manning, C. E., & Morris, E. R. (1991). Conformation and physical properties of the bacterial polysaccharides gellan, welan and rhamsan. In E. Dickinson (Ed.), Food polymers, gels and colloids (pp. 22e33). Cambridge, UK: Royal Society of Chemistry.
- Rodríguez-Hernández, A. I., Durand, S., Garnier, C., Tecante, A., & Doublier, J. L. (2003). Rheologyestructure properties of gellan systems: evidence of network formation at low gellan concentrations. Food Hydrocolloids, 17, 621e628.
- Rolin, C. (1993). Pectin. In R. L. Whistler, & J. N. BeMiller (Eds.), Industrial gums: Polysaccharides and their derivatives (3rd ed.). (pp. 257e293) San Diego, USA: Academic Press.
- Ross-Murphy, S. B. (1984). Rheological methods. In H. W.-S. Chan (Ed.), Biophysical methods in food research. Critical reports on applied chemistry (pp. 195e290). London, UK: SCI.
- Schramm, G. (1981). Optimization of rotovisco tests. Gebruder HAAKE GmbH.
- Suchkov, V. V., Grinberg, V. Y., & Tolstoguzov, V. B. (1981). Steady-state viscosity of the liquid two-phase disperse system waterecaseinesodium alginate. Carbo- hydrate Polymers, 1, 39e53.
- Sworn, G. (2000). Gellan gum. In G. O. Phillips, & P. A. Williams (Eds.), Handbook of hydrocolloids. Cambridge, UK: Woodhead Publishing Ltd.
- Sworn, G., Sanderson, G. R., & Gibson, W. (1995). Gellan gum fluid gels. Food Hydrocolloids, 9, 265e271.
- Syrbe, A., Bauer, W. J., & Klostermeyer, H. (1998). Polymer science concepts in dairy systems, an overview of milk protein and food hydrocolloid interaction. Inter- national Dairy Journal, 8, 179e193.
- Tamime, A. Y., & Marshall, V. M. E. (1997). Microbiology and technology of fermented milks. In B. A. Law (Ed.), Microbiology and biochemistry of cheese and fermented milks (2nd ed.). (pp. 57e152) London: Blackie Academic & Professional.
- Tolstoguzov, V. B. (1986). Functional properties of proteinepolysaccharide mixtures. In J. R. Mitchell, & D. A. Ledward (Eds.), Functional properties of food macro- molecules (pp. 385e415). London, UK: Elsevier.
- Tolstoguzov, V. B. (1991). Functional properties of food proteins and role of pro- teinepolysaccharide interaction. Food Hydrocolloids, 4, 429e468.
- Towler. (1984). Sedimentation in a cultured milk beverage. New Zealand Journal of Dairy Science and Technology, 19, 205e211.
- Tuinier, R., Rolin, C., & de Kruif, C. G. (2002). Electrosorption of pectin onto casein micelles. Biomacromolecules, 3, 632e638.
- Tromp, R. H., de Kruif, C. G., van Eijk, M., & Rolin, C. (2004). On the mechanism of stabilisation of acidified milk drink by pectin. Food Hydrocolloids, 18, 565e572.
- Visser, J., & Voragen, A. G. J. (1996). Pectins and pectinases. In Progress in biotech- nology, Vol. 14. Amsterdam: Elsevier.
- Voragen, A. G. J., Schols, H. A., & Visser, R. G. F. (2003). Advances in pectin and pectinase research. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Walstra, P. (1990). On the stability of casein micelles. Journal of Dairy Science, 73, 1965e1979.
- Willats, W. G. T., Knox, J. P., & Mikkelsen, J. D. (2006). Pectin: new insights into an old polymer are starting to gel. Trends in Food Science & Technology, 17, 97e104.