INORGANIC_CHEMISTRY_EXPERIMENTTION.pdf (original) (raw)
Abstract
Subject: Pure Sodium Chloride from Common Salt materials required dropping funnel, round buttom flask, Erlenmeyer flask, funnel, beaker, glass tube, rubber tube common salt(NaCl), BaCl 2 , (NH 4 ) 2 C 2 O 4 , dye (paranitrobenzen-azo-resorcinol), NaOH,c-H 2 SO 4 ,c-HCl procedure Dissolve 50 grams of common salt in 150 ml of hot water. Cool, filter, and test small portions of the solution qualitatively for sulfate, calcium, and magnesium. To test for sulfate, add barium chloride and dilute hydrochloric acid; for calcium,add ammonium oxalate; for magnesium, add a few drops of a dilute solution of the dye paranitrobenzene-azo-resorcinol followed by 2N sodium hydroxide.In the last test, magnesium gives magnesium hydroxide colored blue by the dye; the blue is distinct from the purple color the dye itself gives with sodium hydroxide. Fig.1. Pure sodium chloride from common salt Place the remaining solution in an Erlenmeyer flask and pass over it a slow stream of hydrogen chloride gas, generated by dropping concentrated sulfuric acid into concentrated hydrochloric acid. To prevent the hydrogen chloride gas from passing out into the room, it is absorbed in sodium hydroxide solution by use of the funnel arrangement shown in Fig.1. Note: (a) The gas is passed over the salt solution and not through it. (b) The funnel dips only a millimeter or so below the surface of the sodium hydroxide. The reasons for these arrangements should be clear.
Figures (8)
Dissolve 50 grams of common salt in 150 ml of hot water. Cool, filter, and test small portions of the solution qualitatively for sulfate, calcium, and magnesium. To test for sulfate, add barium chloride and dilute hydrochloric acid; for calctum,add ammonium oxalate; for magnesium, add a few drops of a dilute solution of the dye paranitrobenzene-azo-resorcinol followed by 2N sodium hydroxide.In the last test, magnesium gives magnesium hydroxide colored blue by the dye; the blue is distinct from the purple color the dye itself gives with sodium hydroxide. from the purple color the dye itself gives with sodium hydroxide. arrangement shown in Fig.1. Note: (a) The gas is passed over the salt solution and not
evaporate slowly in the air. The crystals belong to the monoclinic system. This salt will separate in large well-formed crystals if a cold saturated solution is left to which may be obtained from the side shelf or prepared as a separate exercise.
A saturated solution may be conveniently prepared by the following procedure. In about 500mL of water at 50°C, dissolve somewhat more of the salt whose crystals are to be grown than will dissolve at the expected growing temperature. While stirring vigorously cool the solution to the expected growing temperature (to not cool below this temperature). If crystallization does not take place, add a small crystal to induce it. Stir the suspension of crystals for about 15min and than let the solution stand in contact with the crystals in a covered beaker or stoppered flask in the crystal-growing room for a day or longer. Finally decent the solution from the precipitated crystals. These crystals may be spread out on a piece of filter paper to dry, and among them may be found a suitable seed crystal. A seed crystal must be al single crystal, and so that it may be easily suspended by a thread, it should be least 3mm long. Smaller crystals are not only difficult ot attach to the thread. Save all good seed crystals, for your initial attempt at crystal growing may not be successful. crystal growing may not be successful. seed crystal. A seed crystal must be al single crystal, and so that it may be easily
Comparisions with main-group elements Since the formal maximum oxidation states of the corresponding main group and transitional elements are the same, we may expect some resemblances between them interms of chemical and are readly hydrolysed in water. The elements also form hexa- halo complexes, e.g.[SnCl6]” ,[TiCls]”. Vanadium(V) shows few resemblences to phosphorus(V) , although the tetrahedral oxychlorides VOCI; and POCI; are both liquids and are readly hydrolysed in water . Both chromium(VI) and sulphur(VI) form strongly acidic oxides CrO3 and SO3 , and the covalent oxyhalides CrO2Cl2 and SO2Cl» are easily hydrolysed. Magness(VII) shows some formal resemblances to the halogens in the oxo-compounds Mn2O7, and MnO," Both C1207 and the corresponding manganese compound are powerful oxidizing agents and they are of low stability. The tetrahedral oxyanions ClO, and MnO, are strong oxidizing agents. It must be emphasized, however, that the diffrences between the transitional and main group elements greatly outweigh the similarities mentioned. In particular, the oxidation states of the transition metals that have d eletrons have nocounterpart in the main-group elements. interms of chemical and are readly hydrolysed in water. The elements also form hexa-
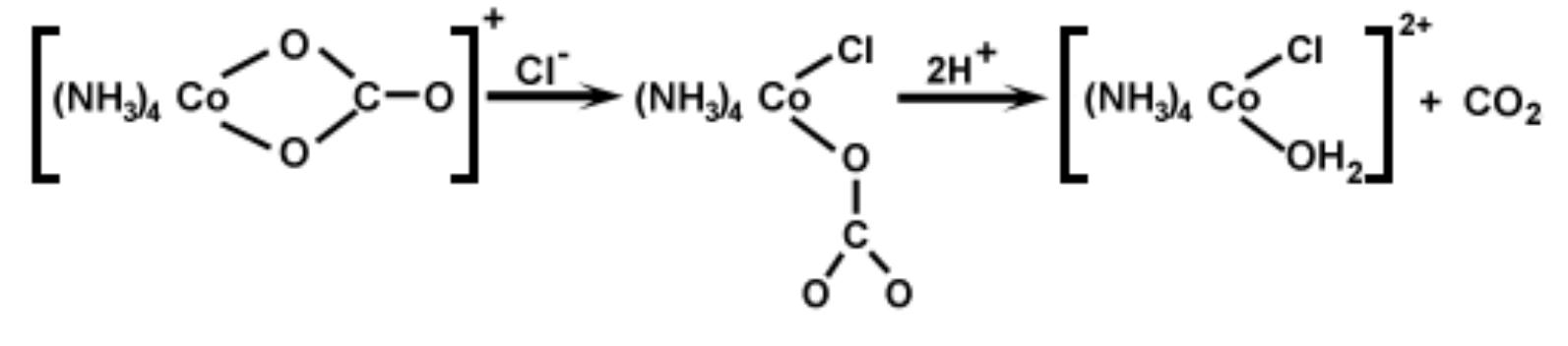
One important method of characterizing ionic substances is the determination of the ability of their solutions to conduct an electric current. Those substances whose solutions have the highest conductivity consist of the greatest number of ions. Thus, < one molar solution of [Co(NH3)4CO3]NO3 will have a lower conductance than < solution of [Co(NH3)sCl]Cl2 of the same concentration. By measuring the conductivity of a solution of a compound, it is possible to determine whethera formula unit of tha compound consists of 2, 3, 4, or more ions. Although measurements will be done or
That O-C bond fission occurs in the intermediate has been established from '*O isotopic exchange studies in several similar reactions of carbonato complexes. The subsequent steps in this preparation involve the substitution of one ligand in the coordination sphere by another. At first glace, one might expect these reactions to proceed according to Sy 1 or Sx2 mechanisms, but even now there is considerable debate as to how these substitutions actually proceed. In Experiment 2, you will have an opportunity to reaction in the preceding sequence probably involves the following mechanism.
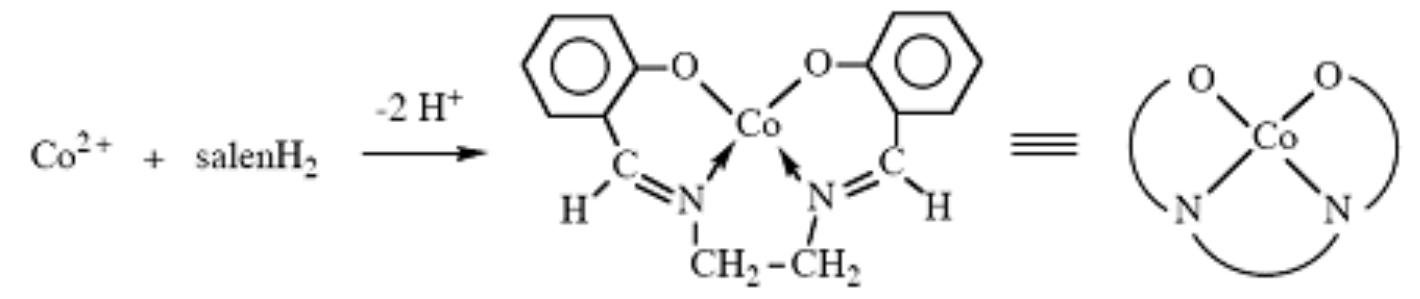
Addition of a Co(II) salt to salenH) results in formation of the Co(II) salen complex: forming five- or six-coordinate complexes. In the solid state it exists in two different forms (I, a dark red isomer inactive toward oxygen binding, and II, the active isomer).
readings gives the amount of O2 absorbed.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.



![Comparisions with main-group elements Since the formal maximum oxidation states of the corresponding main group and transitional elements are the same, we may expect some resemblances between them interms of chemical and are readly hydrolysed in water. The elements also form hexa- halo complexes, e.g.[SnCl6]” ,[TiCls]”. Vanadium(V) shows few resemblences to phosphorus(V) , although the tetrahedral oxychlorides VOCI; and POCI; are both liquids and are readly hydrolysed in water . Both chromium(VI) and sulphur(VI) form strongly acidic oxides CrO3 and SO3 , and the covalent oxyhalides CrO2Cl2 and SO2Cl» are easily hydrolysed. Magness(VII) shows some formal resemblances to the halogens in the oxo-compounds Mn2O7, and MnO," Both C1207 and the corresponding manganese compound are powerful oxidizing agents and they are of low stability. The tetrahedral oxyanions ClO, and MnO, are strong oxidizing agents. It must be emphasized, however, that the diffrences between the transitional and main group elements greatly outweigh the similarities mentioned. In particular, the oxidation states of the transition metals that have d eletrons have nocounterpart in the main-group elements. interms of chemical and are readly hydrolysed in water. The elements also form hexa-](https://figures.academia-assets.com/51980307/table_001.jpg) ](
](![One important method of characterizing ionic substances is the determination of the ability of their solutions to conduct an electric current. Those substances whose solutions have the highest conductivity consist of the greatest number of ions. Thus, < one molar solution of [Co(NH3)4CO3]NO3 will have a lower conductance than < solution of [Co(NH3)sCl]Cl2 of the same concentration. By measuring the conductivity of a solution of a compound, it is possible to determine whethera formula unit of tha compound consists of 2, 3, 4, or more ions. Although measurements will be done or](https://figures.academia-assets.com/51980307/figure_004.jpg) ](
](