The oxidative dissolution mechanism of uranium dioxide. I. The effect of temperature in hydrogen carbonate medium (original) (raw)
Abstract
The oxidative dissolution of uranium (IV) dioxide has been experimentally investigated as a function of hydrogen carbonate concentration at 4 different temperatures (10, 25, 45, and 60°C) by using a continuous thin-layer flow-through reactor. The experimental results have been interpreted as evidence for a bicarbonate-promoted oxidative dissolution mechanism which can be differentiated in to 3 steps: 1) initial oxidation of the uranium dioxide solid surface; 2) binding of HCO 3
FAQs
AI
What is the proposed mechanism for uranium dioxide oxidative dissolution in bicarbonate?add
The study proposes a three-step mechanism: oxidation of U(IV), bicarbonate surface coordination, and product detachment.
How does temperature affect the oxidative dissolution rate of UO2?add
The dissolution rate increases with temperature, displaying an activation energy range of 30-80 kJ mol-1.
What concentrations of hydrogen carbonate were tested in the dissolution experiments?add
Hydrogen carbonate concentrations ranged from 10^-4 to 0.05 mol dm^-3 during the experiments.
How was the experimental dissolution rate of UO2 calculated?add
The rate was calculated using flow rate, uranium concentration, and surface area, expressed in mol m^-2 s^-1.
What discrepancies were noted between predicted and measured dissolution rates?add
Discrepancies suggest that oxidant production by radiolysis affects oxidative dissolution at low concentrations.
Figures (9)
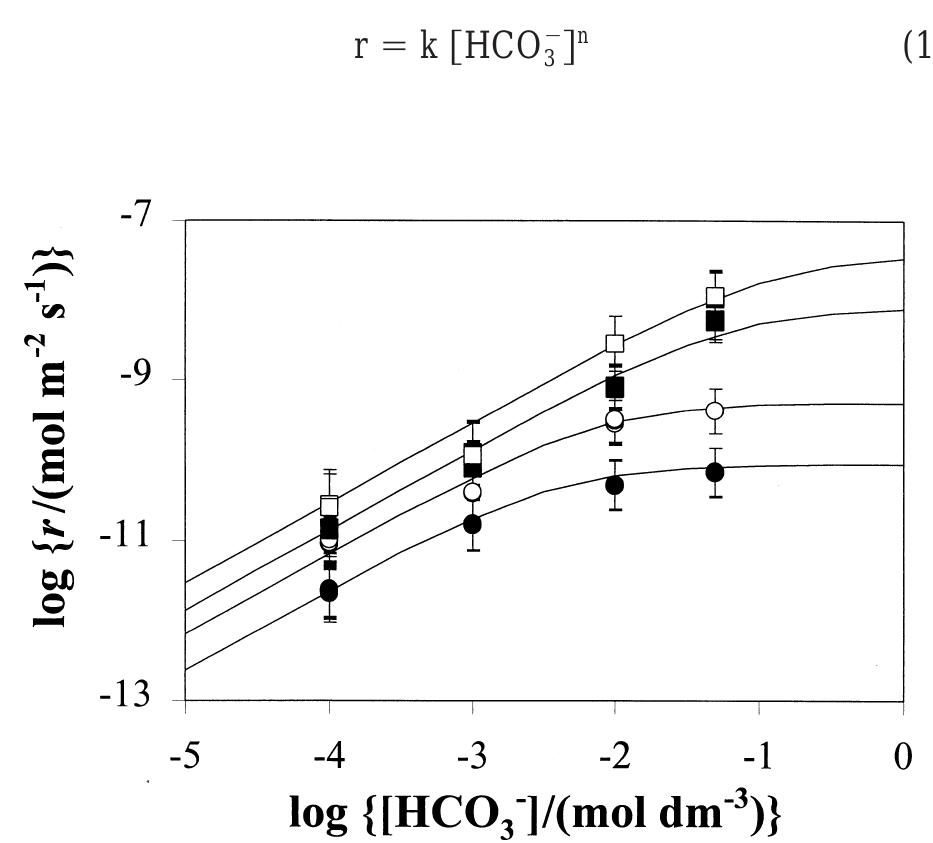
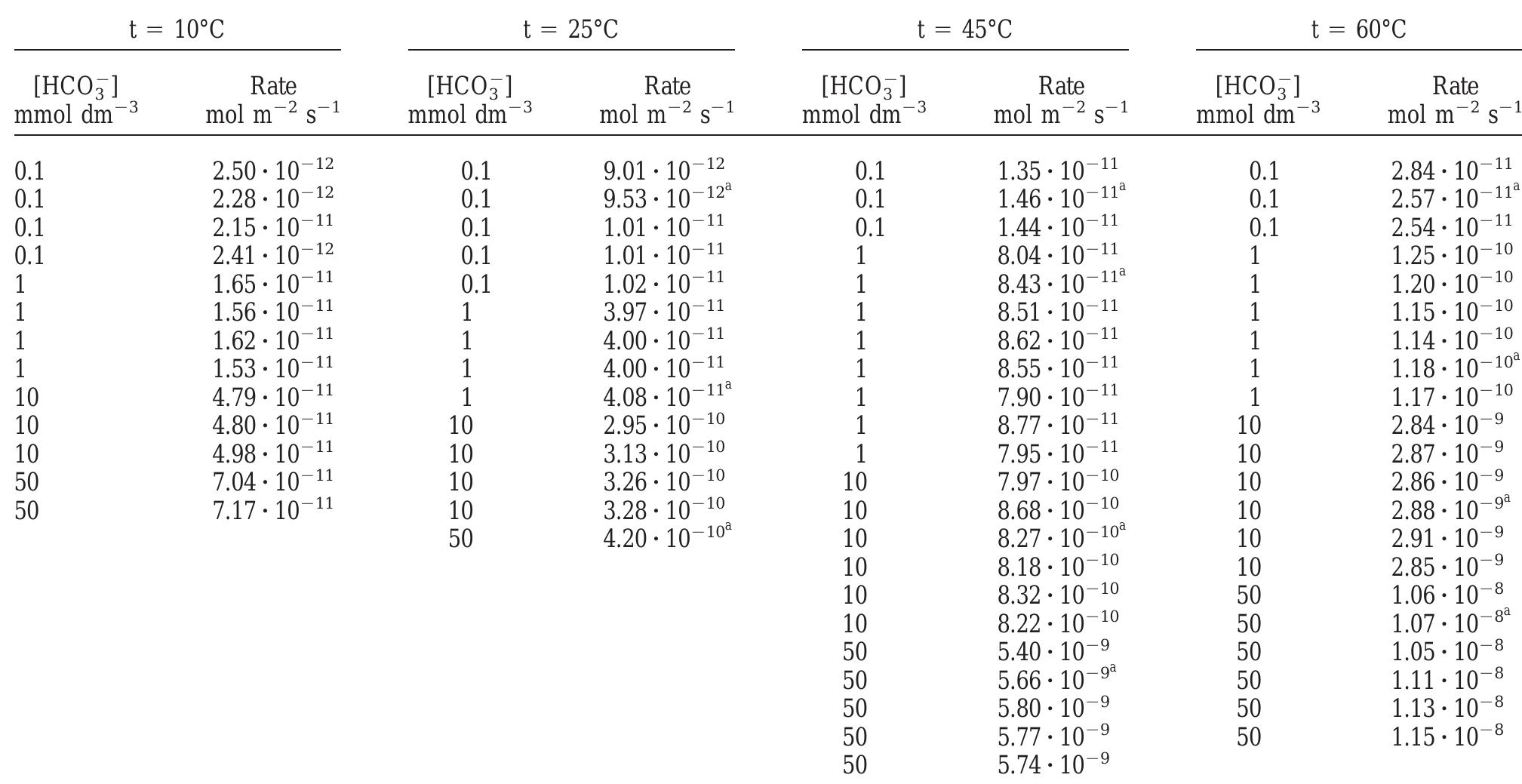
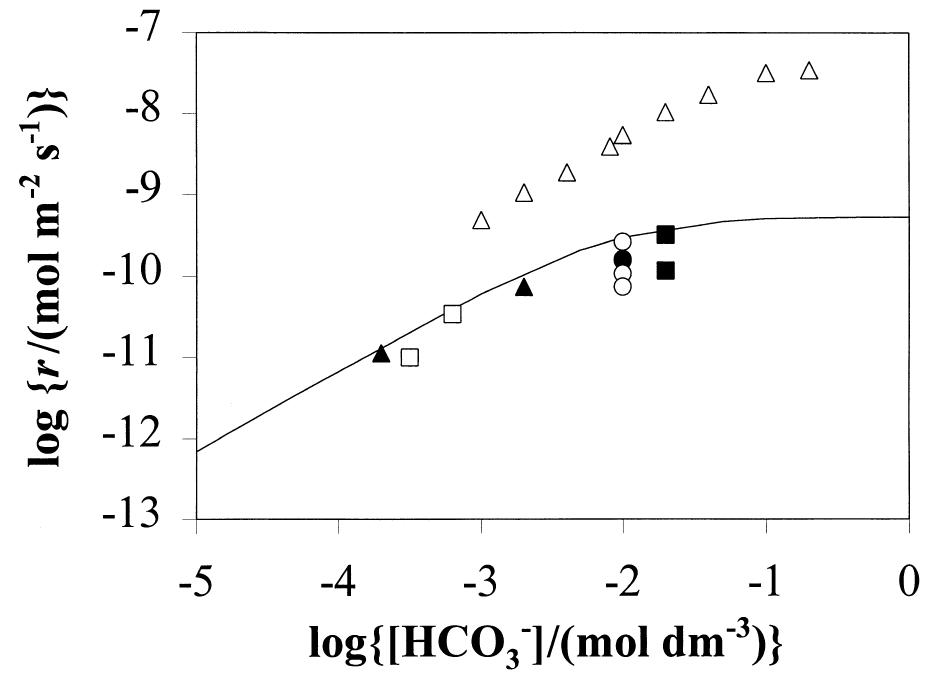
The experimental results in terms of rates of dissolution, tem- perature and bicarbonate concentration are collected in Table 1 and shown in Fig. 1. Preliminary results presented in de Pablo and colleagues (1997) under the same experimental conditions have been also included in Table 1. From these results, an experimental rate equation for the dissolution of UO.(s) in bicarbonate solution can be derived as: Fig. 1. Dissolution rates as a function of bicarbonate concentration at various temperatures compared with the dissolution rates predicted by Eqn. (15) (solid lines) using the constants reported in Table 4. @ 10°C, © 25°C, ™ 45°, 0 60°.
Table 1. Experimental dissolution rates as a function of temperature and bicarbonate concentration. * Data from de Pablo et al. (1997).
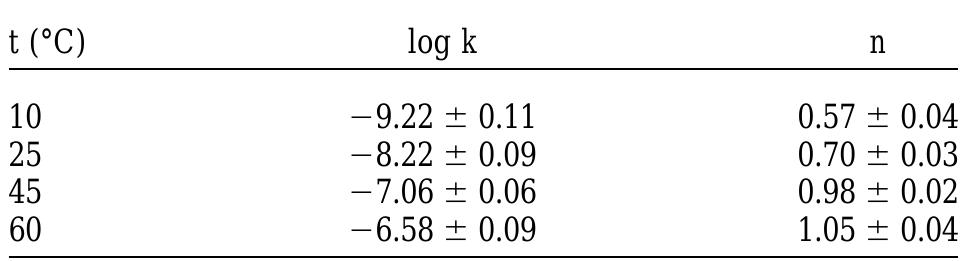
Table 3. Variation of log k and n (see Eqn. 1) with temperature.
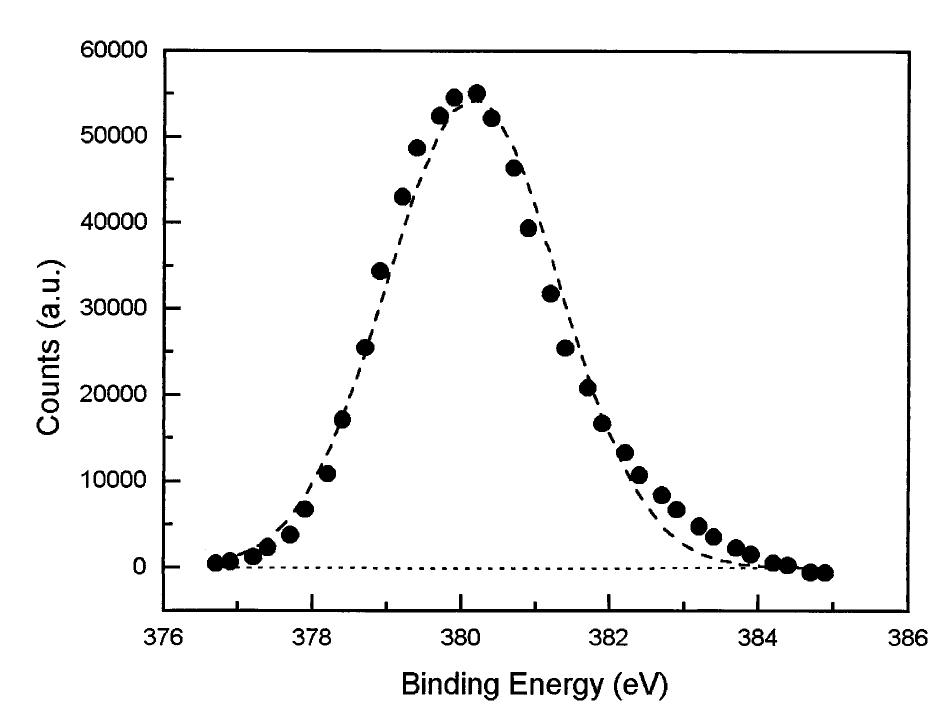
Fig. 3. Peak fit for the Uyg,7/2) peak obtained by X-ray Photoelectron Spectroscopy (XPS). Only a contribution of U(IV) can be observed. The assumption of a fast detachment of the carbonate surface complex (step 3) is supported by results from X-Ray Photo- electron Spectroscopy analysis of surface. In Fig. 3, the fit for the Une7/2) peak of the final solid surface from experiments at 25°C shows that U is observable only in the IV oxidation state, suggesting that the U(VI) remaining at the surface is present only at concentrations below detection, or that it has been rapidly dissolved by the hydrogen carbonate, as previously shown in Bruno and colleagues (1995). The same observation has been reported at acidic pH when the dissolution is enhanced (Sunder et al., 1991; Torrero et al., 1997), while at alkaline pH final solid surface is close to UO. 55, indicating a slower
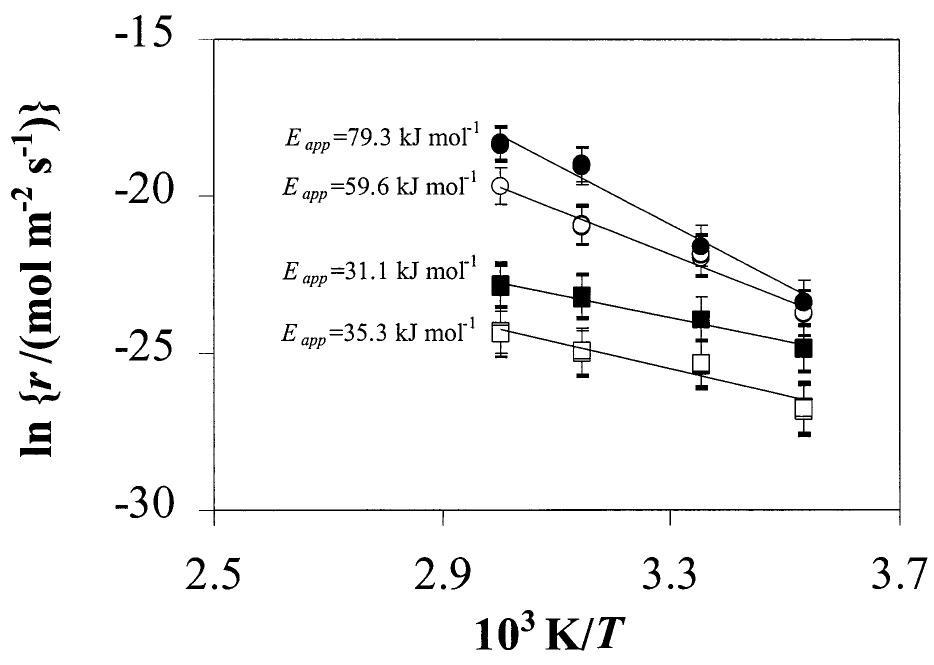
Fig. 2. Dissolution rate as a function of 1/T at various bicarbonate concentrations. From the slopes of these curves, apparent activation energies (E,,,) were obtained by applying the Arrhenius equation. @5-10-? mol dm~* 0 10~? mol dm~*, gi 10~? mol dm~°, 0 10-4 mol dm~3
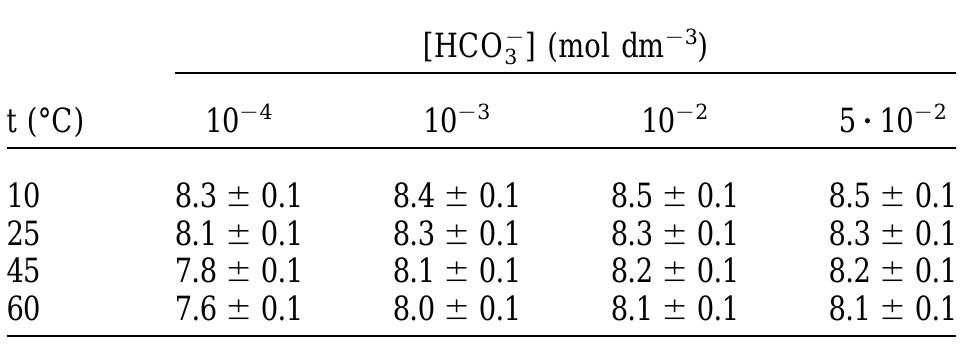
Table 2. pH values at various experimental conditions. where the values of n and k are a function of temperature. The dependence of the rate on pH was not considered because in the pH range of this study (7.5-8.5; see Table 2), it has been proven that dissolution rate is not dependent on pH (Torrero et al., 1997). The values for k and n empirically obtained from the determination of the rate of dissolution are shown in Table 3.
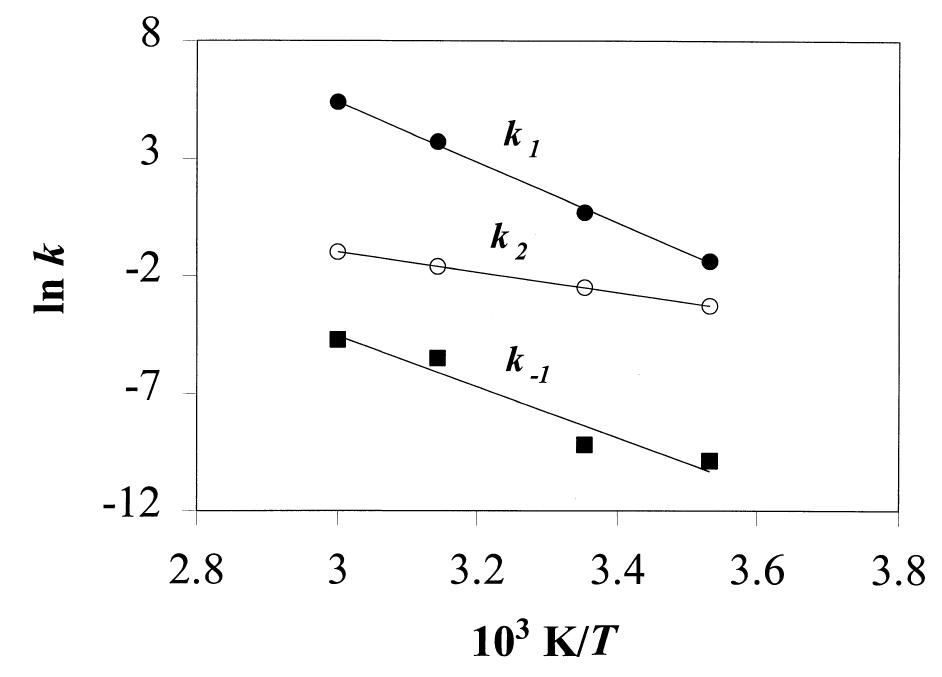
Fig. 4. Kinetic constants as a function of 1/T. From the slopes of these curves, activation energies (E,) were obtained by applying the Arrhenius equation.
Fig. 5. Comparison of the dissolution rates calculated by usinf Eqn. (15) (solid line) and those obtained experimentally by several authors using unirradiated UO, (@, O, MU, A) and natural uraninites (A). @ Bruno and colleagues (1995), O Gray and colleagues (1994), mi Stew- ard and Gray (1994), 1 Grambow (1989), & Gray and Wilson (1995), A Grandstaff (1976).
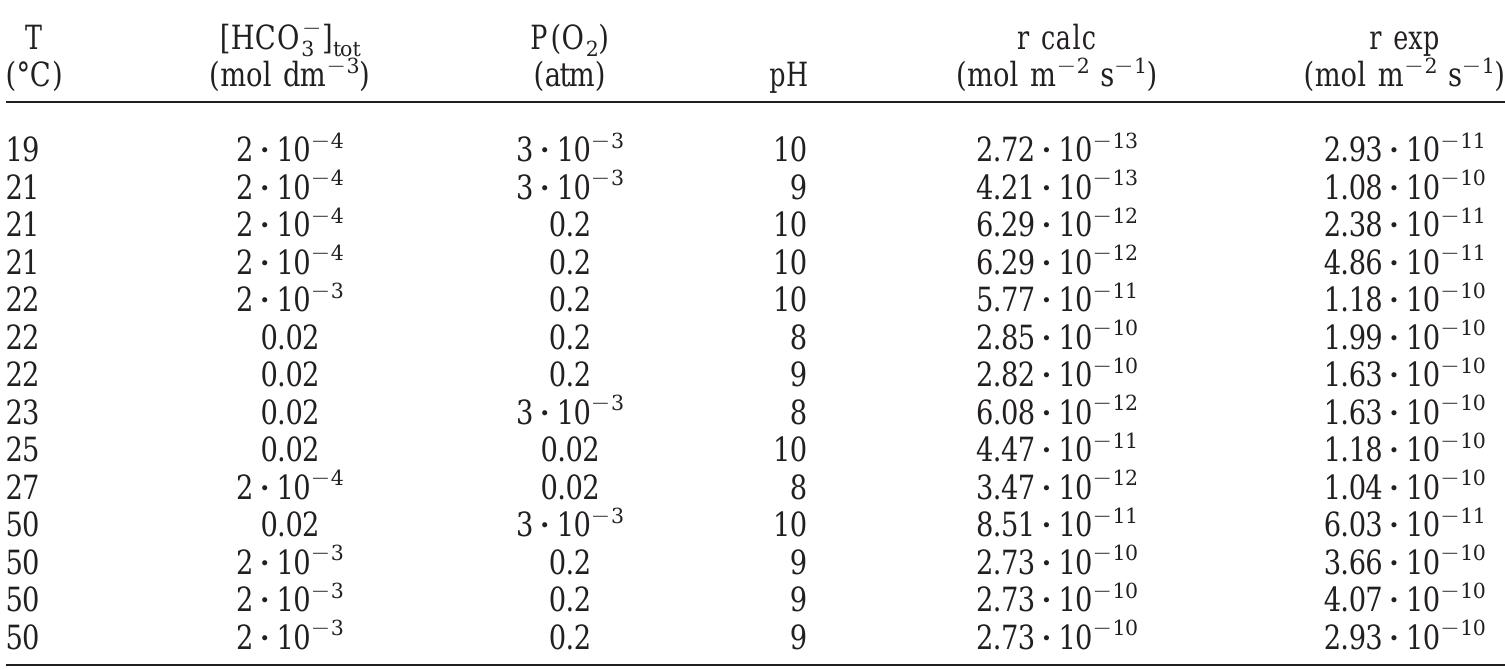
Table 5. Comparison between the dissolution rate calculated with Eqn. (15) and the experimental data obtained by Gray and Wilson (1995). For the rate predictions at 25°C the constants k,, k, and k_, reported in Table 4 have been used. However in all other cases, these constants have been calculated at different temperatures using the E,, values reported in the text.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (28)
- Aronson S., Roof Jr. R. B., and Belle J. (1957) Kinetic study of the oxidation of uranium dioxide. J. Chem. Phys. 27, 137-144.
- Bruno J., Casas I., and Puigdome ´nech I. (1991) The kinetics of disso- lution of UO 2 under reducing conditions and the influence of oxi- dized surface layer (UO 2ϩx ): Application of a continous flow- through reactor. Geochim. Cosmochim. Acta 55, 647-658.
- Bruno J., Casas I., Cera E., de Pablo J., Gime ´nez J., and Torrero M. E. (1995) Uranium (IV) dioxide and simfuel as chemical analogues of nuclear spent fuel matrix dissolution. A comparison of dissolution results in a standard NaCl/NaHCO 3 solution. Mat. Res. Soc. Symp. Proc. 353, 601-608.
- Casas I., Gime ´nez J., Martı ´V., Torrero M. E., and de Pablo J. (1994) Kinetic studies of unirradiated UO 2 dissolution under oxidizing conditions in batch and flow experiments. Radiochim. Acta 66/67, 23-27.
- Davis J. A. and Kent D. B. (1990) Surface complexation modeling in aqueous geochemistry. In Review in Mineralogy: Mineral Water Interface Geochemistry (eds. M. F. Hochella Jr. and A. F. White), Vol. 23, Chap. 5, pp. 177-260. The Mineralogical Society of Amer- ica.
- Pablo J., Casas I., Gime ´nez J., Molera M., and Torrero M. E. (1997) Effect of temperature and bicarbonate concentration on the kinetics of UO 2 (s) dissolution under oxidizing conditions. Mat. Res. Soc. Symp. Proc. 465, 535-542.
- Einziger R. E., Thomas L. E., Buchanan H. C., and Stout R. B. (1992) Oxidation of spent fuel in air at 175 to 195°C. J. Nucl. 190, 53-60.
- Ganor J., Mogollo ´n J. L, and Lasaga A. C. (1995) The effect of pH on kaolinite dissolution rates and on activation energy. Geochim. Cos- mochim. Acta 59, 1037-1052.
- Gevantman L. H. (1995) Solubility of selected gases in water. In Handbook of Chemistry and Physics. 76th Edition (ed. D. R. Lide), pp. 6.1-6.272. CRC Press.
- Grambow B. (1989) Spent fuel, dissolution and oxidation. An evalua- tion of literature data. Report SKB TR 89-13 (Sweden).
- Grambow B., Loida A., Geckeis H., Casas I., Torrero M. E., Gime ´nez J., de Pablo J., and Gago J. (1997) Direct disposal of spent fuel: Chemistry of reactions. In Management and Disposal of Radioactive Waste (ed. T. McMenamin) pp. 224 -237. European Commission.
- Grandstaff D. E. (1976) A kinetic study of the dissolution of uraninite. Geology 8, 1493-1506.
- Gray W. J. and Wilson C. N. (1995) Spent fuel dissolution studies: FY 1991 to 1994. Report PNL-10540 (USA).
- Gray W. J., Leider H. R., and Steward S. A. (1992) Parametric study of LWR spent fuel dissolution kinetics. J. Nucl. Mat. 190, 46 -52.
- Gray W. J., Tait J. C., Steward S. A., and Shoesmith D. W. (1994) Interlaboratory comparison of UO 2 dissolution rates. In High Level Radioactive Waste Management. V Annual International Confer- ence, pp. 2597-2601.
- Hiskey J. B. (1979) Kinetics of uranium dioxide dissolution in ammo- nium carbonate. Trans. Inst. Nin. Metall. Sect. C 88, C145-C152.
- Lasaga A. C. (1984) Chemical kinetics of water-rock interactions. J. Geophys. Res. 89, 4009 -4025.
- Nicholson R. V., Gillham R. W., and Reardon E. J. (1988) Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 52, 1077-1085.
- Posey-Dowty J., Axtmann E., Crerar D., Borcsik M., Ronk A., and Woods W. (1987) Dissolution rate of uraninite and uranium roll- front ores. Economic Geology 82, 184 -194.
- Puigdome ´nech I., Rard J. A., Plyasunov A. V., and Grenthe I. (1997) Temperature corrections to thermodynamic data and entalphy calcu- lations. In Modelling in Aquatic Chemistry (eds. I. Grenthe and I. Puigdomenech), Chap. 10, pp. 427-493. OECD NEA.
- Robbins J. C. (1978) Field techniques for the measurement of uranium in natural waters, Can. Inst. Min. Metall. Bull. 71, 61-67.
- Schortmann W. E. and DeSesa M. A. (1958) Kinetics of the dissolution of uranium dioxide in carbonate-bicarbonate solutions. Proc. Second United Nation International Conference on the Peaceful Uses of Atomic Energy 3, 333-341.
- Shoesmith D. W. and Sunder S. (1992) The prediction of nuclear fuel (UO 2 ) dissolution rates under waste disposal conditions J. of Nucl. Mat. 190, 20 -35.
- Shoesmith D. W., Sunder S., Bailey M. G., and Wallace G. J. (1989) The corrosion of nuclear fuel (UO 2 ) in oxygenated solutions. Cor- rosion Sci. 29, 1115-1128.
- Sunder S., Shoesmith D. W., Lemire R. J., Bailey M. G., and Wallace G. J. (1991) The effect of pH on the corrosion of nuclear fuel (UO 2 ) in oxygenated solutions. Corros. Sci. 32, 373-386.
- Steward S. A. and Gray W. J. (1994) Comparison of uranium disso- lution rates from spent fuel and uranium dioxide. High Level Radio- active Waste Management. V Annual International Conference, 2602-2608.
- Thomas G. F. and Till G. (1984) The dissolution of unirradiated UO 2 fuel pellets under simulated disposal conditions. Nucl. Chem. Waste Manag. 5, 141-147.
- Torrero M. E., Baraj E., de Pablo J., Gime ´nez J., and Casas I. (1997) Kinetics of corrosion and dissolution of uranium dioxide as a func- tion of pH. Int. J. Chem. Kinet. 29, 261-267.