The relationship between dental metal allergy, periodontitis, and palmoplantar pustulosis: An observational study (original) (raw)
Abstract
Metal alloys have long been used in the dental profession. Although a range of acrylic materials have been introduced recently, metal alloys are still widely used in dental prostheses. Dental metal allergy refers to the symptoms of contact dermatitis, which is suspected to be associated with the use of metal alloys in dental treatment; however, it does not necessarily reflect a causal relationship. Since being reported in 1928, several clinical, in vitro, and animal studies have investigated its prevalence and etiology[1]. Nevertheless, the cause of dental metal allergy remains poorly understood, despite a recent increase in the number of affected patients in Japan. Although the pathophysiological mechanisms remain unclear, several skin diseases have been suggested to be associated with dental metal alloys, including palmoplantar pustulosis (PPP), dyshidrotic eczema, contact dermatitis, and oral lichen planus[2-6]. Specific types of dermatitis have also been reported to be associated with periodontal disease and apical periodontitis[2,4,7-11]. Studies suggest that the treatment of chronic inflammation, and the replacement of dental metals present in the prostheses may result in the improvement of the skin diseases. This study aimed to determine the relationship between dental metal allergy, PPP, and periodontitis among a group of patients from a dental metal allergy clinic. 2. Materials and Methods 2.1. Patients This study included 436 patients who visited our dental metal allergy clinic between April 1, 2009, and March 31, 2016. All patients provided signed informed consent to participate in the clinical study and to undergo dental treatment, patch testing, and electron probe microanalysis (EPMA). Patients who did not consent to the examination or inclusion in the clinical study, or received a diagnosis that did not require further examination, were excluded. The study protocol was approved by the Research Ethics Committee of our university (2009-5044) and was performed in accordance with the Declaration of Helsinki.
Figures (13)
Aq: Purified water, Pet: Petrolatum Table 1. Reagents of the dental metal series used in the patch test
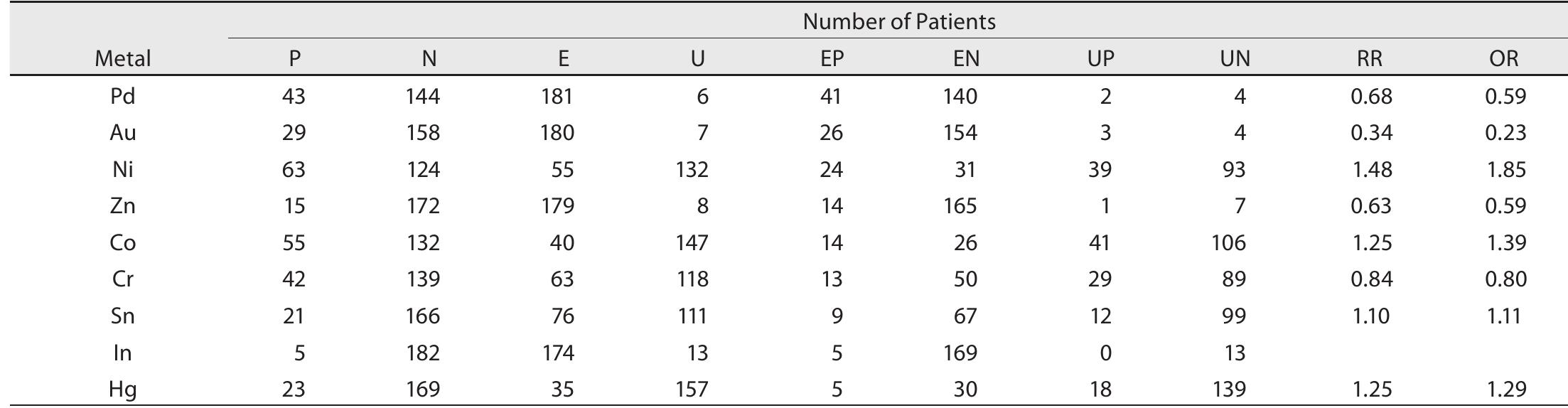
Table 2. Contingency table for the estimation of the relative risk for suspect: ed allergenic metal analyzed using EPMA (EPMA-1610, Shimadzu, Japan). In cases involv- ing a single prosthesis, such as the crown, inlay, or amalgam filling, a single metal sample was collected. In cases involving removable or fixed partial dentures, metal samples were collected from each com- ponent, such as the clasp, bar, rest, soldering, and crown. In cases requiring the replacement of the metal prosthesis with a cast post and core, the samples were collected from the cast post and core.
Fig. 1. Age distribution of patients who visited our clinic.
Fig. 2. Flowchart of classification for patients with dental metal allergy. Table 4. Skin disease distribution among all patients assessed
PPP, palmoplantar pustulosis Table 3. Contingency table for estimating the relative risk and odds ratio be- tween PPP and metal allergy or periodontitis.
Fig. 4. Results of the electron probe microanalysis (EPMA) for metals con- tained in dental prostheses.
Fig. 3. Results of the patch tests. The graph shows the number of positive tests for each metal reagent tested.
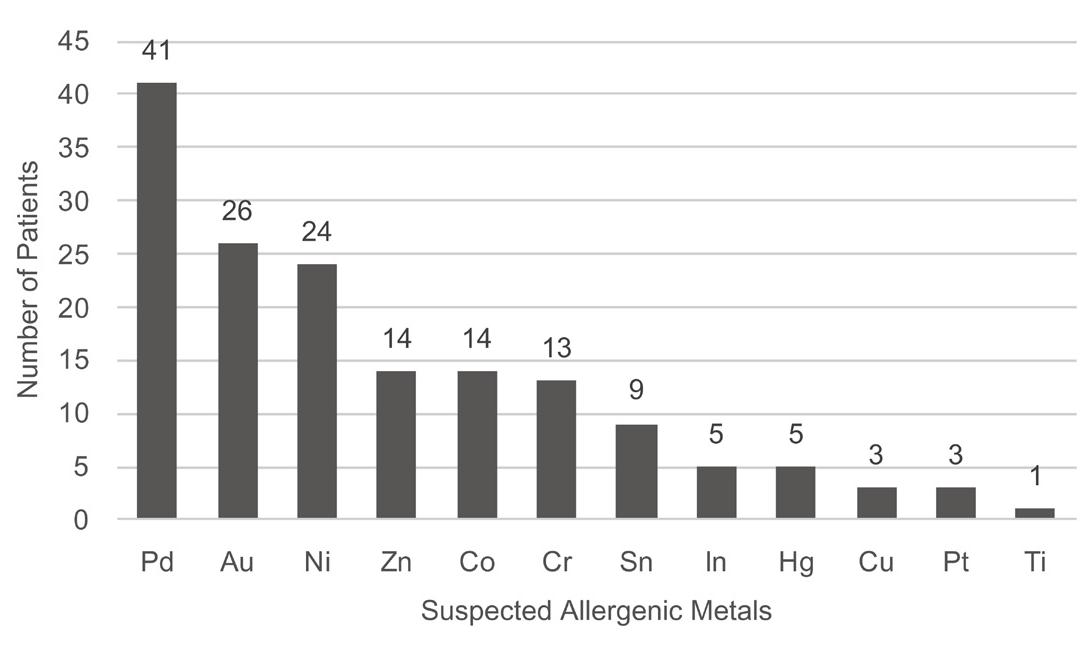
Fig. 5. Prevalence of suspected allergenic metals in the dental prostheses.
P: Patients that tested positive to the patch test for the suspected allergenic metal. N: Patients that tested negative to the patch test for the suspected allergenic metal. E: Exposed to the suspected allergenic metal. U: Not exposed to the suspected allergenic metal. EP: E and P, EN: Eand N UP: U and P UIN-llandN Table 5. Relative risk and odds ratio of dental metal allergy
Fig. 6. Age distribution of patients with palmoplantar pustulosis.
Fig. 7. Flowchart of classification for PPP patients with dental metal allergy and periodontitis.
Fig. 8. Prevalence of periodontitis among patients with palmoplantar pus tulosis.
Fig. 9. Prevalence of periodontitis among patients aged 45-54 years with palmoplantar pustulosis.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (23)
- Fleischmann P. Zur Frage der Gefährlichkeitkleinster Quecksilbermengen 1 ). DMW -Deutsche Medizinische Wochenschrift. 1928;54:304-7. https://doi. org/10.1055/s-0028-1125056 German
- Kitagawa M, Murakami S, Akashi Y, Oka H, Shintani T, Ogawa I, et al. Current status of dental metal allergy in Japan. J Prosthodont Res. 2019;63:309-12. https://doi.org/10.1016/j.jpor.2019.01.003, PMID:30738702
- Murai O, Sasaki D, Ando Y, Fujimura A, Oikawa H, Suwa N, et al. Improve- ment of pustulosis palmaris et plantaris by periodontal infection control in a patient with chronic periodontitis. Clin Lab. 2012;58:323-7. PMID:22582507
- Kikuchi N, Yamamoto T. Dental infection as a triggering factor in pal- moplantar pustulosis. Acta Derm Venereol. 2013;93:721-2. https://doi. org/10.2340/00015555-1552, PMID:23462950
- Song H, Yin W, Ma Q. Allergic palmoplantar pustulosis caused by cobalt in cast dental crowns: a case report. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2011;111:e8-10. https://doi. org/10.1016/j.tripleo.2010.12.013, PMID:21439867
- Liu F, Zhang M, Lou Y, Liu H, Sang H. The spontaneous regression of pal- moplantar pustulosis following removal of dental amalgams: A report of two cases. Australas J Dermatol. 2016;57:e93-6. https://doi.org/10.1111/ ajd.12366, PMID:26081174
- Kosugi M, Ishihara K, Okuda K. Implication of responses to bacte- rial heat shock proteins, chronic microbial infections, and dental metal allergy in patients with pustulosis palmaris et plantaris. Bull Tokyo Dent Coll. 2003;44:149-58. https://doi.org/10.2209/tdcpublication.44.149, PMID:14694830
- Kouno M, Nishiyama A, Minabe M, Iguchi N, Ukichi K, Nomura T, et al. Retrospective analysis of the clinical response of palmoplantar pustulosis after dental infection control and dental metal removal. J Dermatol. 2017;44:695-8. https://doi.org/10.1111/1346-8138.13751, PMID:28150339
- Morimoto M, Asaka T, Kamaguchi M, Yamashita E, Sakata K, Ohuchi M, et al. A study on the involvement of dental metal allergy and dental focal infec- tion in palmoplantar pustulosis. Japanese Journal of Oral and Maxillofacial Surgery. 2019;65:447-54. https://doi.org/10.5794/jjoms.65.447
- Ishihara K, Ando T, Kosugi M, Kato T, Morimoto M, Yamane G, et al. Relation- ships between the onset of pustulosis palmaris et plantaris, periodontitis and bacterial heat shock proteins. Oral Microbiol Immunol. 2000;15:232-7. https://doi.org/10.1034/j.1399-302x.2000.150404.x, PMID:11154408
- Akiba Y, Tomizuka K, Kaku M, Kawasaki M, Nagasawa M, Takano R, et al. Analysis of patients visiting Niigata University Medical and Dental Hospital with chief complaints of metal allergy and/or focal infection in the previous 8 years. The Indonesian Journal of Dental Research. 2015;1:109-15. https:// doi.org/10.22146/theindjdentres.10002
- Ministry of Health, Labour and Welfare Report on the Survey of Dental Diseases in Japan. 2016. https://www.mhlw.go.jp/toukei/list/dl/62-28-02\. pdf
- Masui Y, Ito A, Akiba Y, Uoshima K, Abe R. Dental metal allergy is not the main cause of palmoplantar pustulosis. J Eur Acad Dermatol Venereol. 2019;33:e180-1. https://doi.org/10.1111/jdv.15434, PMID:30653749
- Lachapelle JM, Maibach HI. Patch testing and prick testing: a practical guide official publication of the ICDRG. Switzerland AG, Springer Nature; 2019. https://doi.org/10.1007/978-3-030-27099-5
- Fregert S. Manual of contact dermatitis: On behalf of the International Contact Dermatitis Research Group and the North American Contact Der- matitis Group. 2nd ed. Copenhagen: Munksgaard Publishers; 1981.
- Greenland S, Thomas DC, Morgenstern H. The rare-disease assumption revisited. A critique of "estimators of relative risk for case-control studies". Am J Epidemiol. 1986;124:869-76. https://doi.org/10.1093/oxfordjournals. aje.a114476, PMID:3776970
- Inoue M. The Status Quo of the Metal Allergy and the Measures Against it in Dentistry. Nippon Hotetsu Shika Gakkai Zasshi. 1993;37:1127-38. https://doi. org/10.2186/jjps.37.1127
- Ahlström MG, Thyssen JP, Wennervaldt M, Menné T, Johansen JD. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019;81:227-41. https://doi.org/10.1111/cod.13327, PMID:31140194
- Schmidt M, Raghavan B, Müller V, Vogl T, Fejer G, Tchaptchet S, et al. Crucial role for human Toll-like receptor 4 in the development of contact al- lergy to nickel. Nat Immunol. 2010;11:814-9. https://doi.org/10.1038/ni.1919, PMID:20711192
- Lidén C, Andersson N, Julander A, Matura M. Cobalt allergy: suitable test concentration, and concomitant reactivity to nickel and chromium. Contact Dermat. 2016;74:360-7. https://doi.org/10.1111/cod.12568, PMID:26996152
- Fowler JF Jr. Cobalt. Dermatitis. 2016;27:3-8. https://doi.org/10.1097/ DER.0000000000000154, PMID:26756508
- Bonefeld CM, Nielsen MM, Vennegaard MT, Johansen JD, Geisler C, Thyssen JP. Nickel acts as an adjuvant during cobalt sensitization. Exp Dermatol. 2015;24:229-31. https://doi.org/10.1111/exd.12634, PMID:25580744
- Brunasso Vernetti AMG, Puntoni M, Massone C. Palmoplantar pustulosis and allergies: A systematic review. Dermatol Pract Concept. 2019;9:105-10. https://doi.org/10.5826/dpc.0902a05, PMID:31106012