Vaccine potentiality of different antigenic preparations of Aeromonas hydrophila in Rohu, Labeo rohita Ham (original) (raw)
Abstract
To develop vaccine for rohu (Labeo rohita), efficacy of three antigenic preparations from Aeromonas hydrophila were evaluated. Thirty six tanks with ten rohu were divided in quadruplicates (R1 to R4) with nine tanks (G1 to G9). Rohu of G1 to G6 tanks were given intraperitoneal vaccine with outer membrane protein, somatic protein and formalin-inactivated whole cell itself and along with Incomplete Freund’s Adjuvant @ 200 µg/fish, G7 and G8 tanks were injected with Incomplete Freund’s Adjuvant (100 µl/fish) and normal saline (100 µl/fish) respectively and G9 tanks were kept as control. After 28 d, rohu of R3 and R4 were subjected to intramuscular A. hydrophila challenge (LD50) @ 2.85×106 cells/fish for 7 d and RPS (%) was calculated. Specific cellular and humoral immune responses were determined for rohu of R1 and R2. Results showed that rohu immunized with outer membrane protein along with adjuvant could offer an appropriate vaccine strategy.
Key takeaways
AI
- Outer membrane protein antigen with Incomplete Freund's Adjuvant showed 81.81% relative percentage survival (RPS).
- The study evaluates three antigenic preparations for vaccine development against Aeromonas hydrophila in rohu.
- Rohu immunized with outer membrane protein exhibited the highest humoral and cellular immune responses.
- Control groups exhibited significant mortality with 70-90% showing severe clinical signs post-challenge.
- A total of 36 tanks with 10 rohu each were used for the experiment, divided into quadruplicates.
Figures (4)
Dr. Gadadhar Dash Professor, Department of Aquatic Animal Health, Faculty of Fishery Sciences, West Bengal University of Animal and Fishery Sciences, Chakgaria, Panchasayar, Kolkata, West Bengal, India
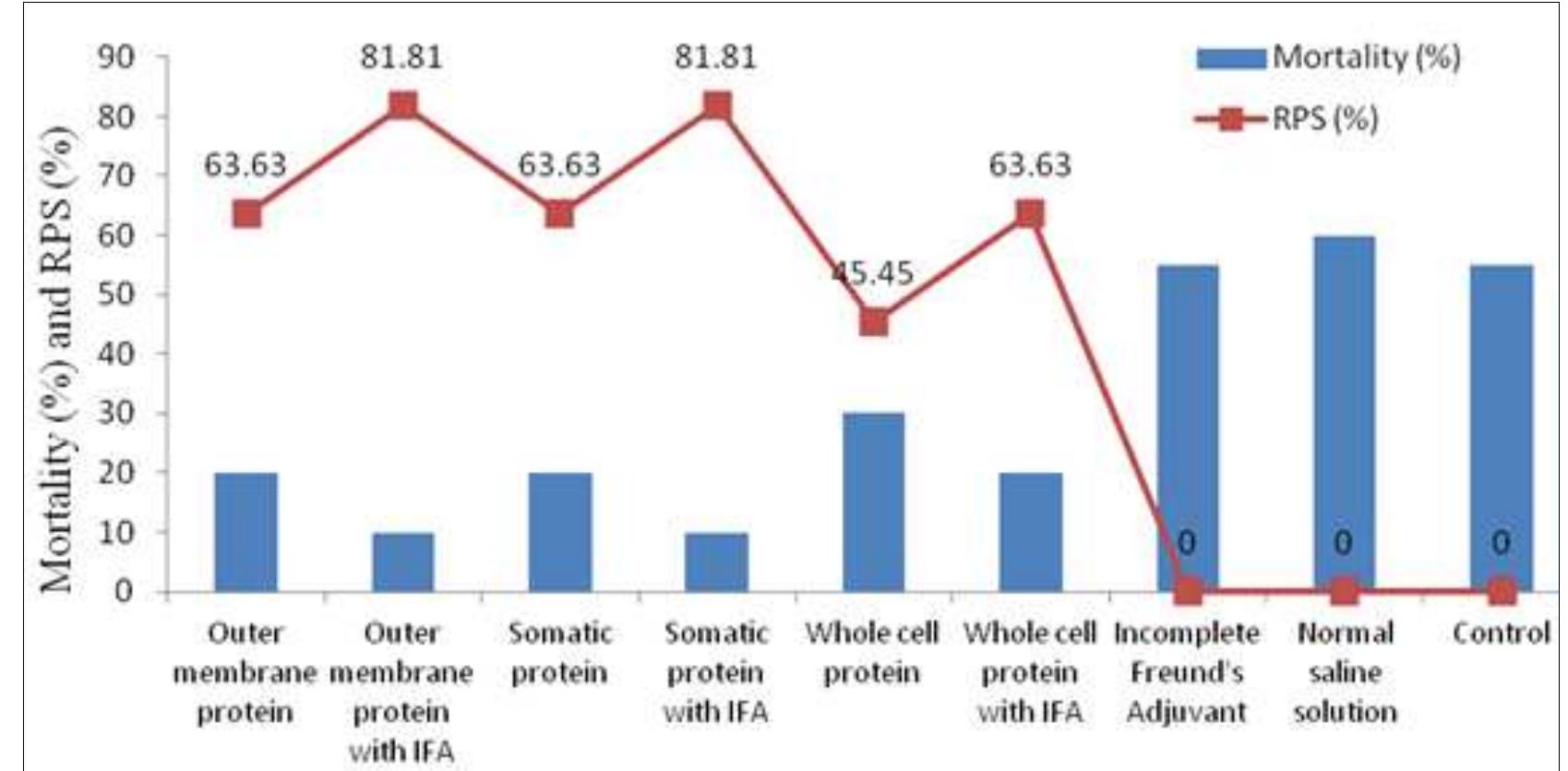
Fig 1: Mortality (%) & RPS (%) after 7 d A. hydrophila challenge of vaccinated rohu 3.3. Gross clinical signs, mortality and relative percentage of survival (%) during experiment: No mortality was observed in all tanks (G; to Go) of Ri to Ry groups during 28 days of the experiment except few clinical signs. Few pinpoint hemorrhages were noticed in only thirteen rohu out of all groups of quadruplicates tank injected with outer membrane protein antigen, somatic protein antigen, whole cell protein antigen itself and whole cell protein antigen along with Freund’s incomplete adjuvant. Those gross clinical changes observed during 28-days vaccination might be due to the pathogenic effects of virulent A. hydrophila N10P. During 7 dA. hydrophila challenge for R3 and Ry groups, 81.81% RPS (Fig.l) was found in case of fishes vaccinated with both outer membrane protein antigen and somatic protein antigen mixed with equal volume of Incomplete Freund’s Adjuvant. In both cases, less than 25% of rohu showed minute clinical signs like ulceration patches on body. Fishes immunized with outer membrane protein antigen, somatic protein antigen itself and whole-cell protein antigen along with Incomplete Freund’s Adjuvant showed 63.63% RPS (Fig.1) with clinical signs like pinpoint hemorrhages and ulceration patches on body for less than 30% rohu. Whereas, 45.45% RPS (Fig. 1) were noticed in case of fishes injected with whole-cell protein antigen itself but number of fishes clinically infected was 45% of population. Relative percentage of survival was found nil for the Incomplete Freund’s Adjuvant injected, normal saline solution injected and control rohu where 70-90% of fishes showed extensive clinical signs with head lesion, fin rot and tail rot, ulceration and hemorrhages on skin with 55-60% mortality. Our results differed from the observations of Sen e: al. 81 where they observed highest relative percentage survival in formalin killed whole-cell A. hydrophila along with adjuvant vaccinated rohu after 60 days post-challenge. Sun et al. °°! observed highest relative percent survival in A. hydrophila immunized grass carp (Ctenopharyngodon idella) in the Lipopolysaccharide and outer membrane protein injected groups (83.3% and 72.2%, respectively) but 90% fish died after challenge in phosphate buffer saline injected control group.
Table 1: OD values after 28" d post vaccination (Ri and R2) as assessed by ELISA [Data (n=3) are presented as mean+standard deviation. Values with different lower case letter superscripts differ significantly (P< 0.05) between the control and different treatment within the same column. Values with different upper case letter superscripts differ significantly (P< 0.05) between in vitro humoral immunity assessment against homologous and heterologus antigen cross-response within the same vaccinated groups. ]
Table 2: Stimulation index (SI) values after 28 d of immunization

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (33)
- References
- Bastardo A, Ravelo C, Castro N, Calheiros J, Romalde JL. Effectiveness of bivalent vaccines against Aeromonas hydrophila and Lactococcus garvieae infections in rainbow trout Oncorhynchus mykiss (Walbaum). Fish and Shellfish Immunology. 2012; 32:756-761. https://doi.org/10.1016/j.fsi.2012.01.028
- Bera A, Joardar SN, Abraham TJ, Batabyal S. Dynamic changes in specific immune-effector activities in Aeromonas hydrophila sensitized Catla, Catla catla (Hamilton). Indian Journal of Comparative Microbiology Immunology and Infectious Diseases. 2010; 31:5-10.
- Bharadwaj A, Abraham TJ and Joardar SN. Immune effector activities in challenged rohu, Labeo rohita after vaccinating with Aeromonas bacterin. Aquaculture. 2013; 392:16-22. http://dx.doi.org/10.1016/j.aquaculture.2013.01.016
- Bøgwald J. and Dalmo RA. Review on immersion vaccines for fish: An Update 2019. Microorganisms. 2019; 7(12):1-28. https://doi.org/10.3390/microorganisms7120627
- Chandran MR, Aruna BV, Logambal SM and Michael RD. Immunization of Indian major carps against Aeromonas hydrophila by intraperitoneal injection. Fish and Shellfish Immunology. 2002; 13:1-9. https://doi.org/10.1006/fsim.2001.0374
- Das P, Joardar SN, Abraham TJ, Kamilya D and Batabyal S. Dynamic changes in immune-effector characteristics of Indian major carp, rohu (Labeo rohita) sensitized with Aeromonas hydrophila. Indian Journal of Comparative Microbiology Immunology and Infectious Diseases. 2009; 30:45-49.
- Dash P, Sahoo PK, Gupta PK, Garg LC, Dixit A. Immune responses and protective efficacy of recombinant outer membrane protein R (rOmpR)-based vaccine of Aeromonas hydrophila with a modified adjuvant formulation in rohu (Labeo rohita). Fish and Shellfish Immunology. 2014; 39(2):512-523. https://doi.org/10.1016/j.fsi.2014.06.007
- Dash S, Das SK, Samal J, Ojha PK, Patra JK and Thatoi H. Dose dependence specific and non-specific immune responses of Indian major carp (L. rohita Ham) to intraperitoneal injection of formalin killed Aeromonas hydrophila whole cell vaccine. Veterinary Research Communications. 2011; 35:541-552. https://doi:10.1007/s11259-011-9498-2
- Dubey S, Avadhani K, Mutalik S, Sivadasan SM, Maiti B, Paul J, Girisha SK et al. Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose- dependent protective immunity in rohu (Labeo rohita). Vaccines. 2016; 4(2):21. https://doi.org/10.3390/vaccines4020021
- Fang HM, Ling KC, Ge R and Sin YM. Enhancement of protective immunity in blue gourami, Trichogaster trichopterus (Pallas), against Aeromonas hydrophila and Vibrio anguillarum by A. hydrophila major adhesin. Journal of Fish Diseases. 2000; 23:137-145. https://doi.org/10.1046/j.1365-2761.2000.00229.x
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in action, 2020. Rome. https://doi.org/10.4060/ca9229en
- Gudding R, Van Muiswinkel WB. A history of fish vaccination: science-based disease prevention in aquaculture. Fish and Shellfish Immunology. 2013; 35(6):1683-1688. https://doi.org/10.1016/j.fsi.2013.09.031
- Hu M, Wang N, Pan ZH, Lu CP, Liu YJ. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Letters in Applied Microbiology. 2012; 55:224-223. https://doi.org/10.1111/j.1472-765X.2012.03281.x
- Jayasankar P. Present status of freshwater aquaculture in India -A review. Indian Journal of Fisheries. 2018; 65(4):157-165. https://doi.org/10.21077/ijf.2018.65.4.81300-20
- Kamilya D, Maiti TK, Joardar SN, Mal BC. Adjuvant effect of mushroom glucan and bovine lactoferrin upon Aeromonas hydrophila vaccination in catla, Catla catla (Hamilton). Journal of Fish Diseases. 2006; 29:331-337. https://doi.org/10.1111/j.1365-2761.2006.00722.x
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of biological chemistry. 1951; 193:65-275.
- Maji S, Mali P, Joardar SN. Immunoreactive antigens of the outer membrane protein of Aeromonas hydrophila, isolated from goldfish, Carassius auratus (Linn.). Fish and Shellfish Immunology. 2006; 20:462-473. https://doi:10.1016/j.fsi.2005.06.003
- Mali P, Maji S, Joardar SN. Antigenic characterization of outer membrane protein of Aeromonas sobria isolated from Goldfish (Carassius auratus L.). The Israeli Journal of Aquaculture -Bamidgeh. 2007; 59(2):91-98.
- Mzula A, Wambura PN, Mdegela RH, Shirima GM. Current state of modern biotechnological-based Aeromonas hydrophila vaccines for aquaculture: a systematic review, BioMed Research International, Article ID 3768948, 2019, 1-11.
- Ouchterlony O. Antigen antibody and reactious in gels. Acta Pathologica at Microbiologica Scandinavia. 1949; 26:507-515.
- Plumb JA, Milroy R, Kaye SB. Effects of the pH Dependence of 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium Bromide-Formazan Absorption on Chemosensitivity Determined by a Novel Tetrazolium- based Assay. Cancer Research. 1989; 49:4435-4440.
- Rasmussen-Ivey CR, Hossain, MJ, Odom SE, Terhune JS, Hemstreet WG, Shoemaker CA et al. Classification of a hypervirulent Aeromonas hydrophila pathotype responsible for epidemic outbreaks in warm-water fishes. Frontiers microbiology. 2016; 7:1615. https://doi.org/10.3389/fmicb.2016.01615
- Reed LJ, Muench H. A simple method of estimating fifty per cent end points. American Journal of Hygiene. 1938; 27:493-497.
- Sahoo C, Joardar SN. Potential use of enzyme conjugated anti-carp immunoglobulin in detecting specific antibodies in carp. Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases. 2004; 25:37-40.
- Sahoo PK, Mukherjee SC, Nayak SK, Dey S. Acute and subchronic toxicity of aflatoxin B1 to rohu, Labeo rohita (Hamilton). Indian Journal of Experimental Biology. 2001; 39:453-458.
- Saikia D, Kamilya D. Immune responses and protection in catla (Catla catla) vaccinated against epizootic ulcerative syndrome. Fish and Shellfish Immunology. 2012; 32:353-359. https://doi.org/10.1016/j.fsi.2011.11.030
- Sardar P, Joardar SN, Ganesan P, Abraham TJ. Seroreactivity of somatic soluble proteins of Vibrio alginolyticus. Indian Journal of Fisheries. 2004; 51:239- 244.
- Sen SS, Giri SS, Sukumaran V. Immune responses and protection in rohu vaccinated against Aeromonas hydrophila infection. Aquaculture International. 2014; 22:1637-1648.
- Shoemaker CA, Klesius PH, Evans JJ, Arias CR. Use of modified live vaccines in aquaculture. Journal of World Aquaculture Society. 2009; 40(5):573-585. https://doi.org/10.1111/j.1749-7345.2009.00279.x
- Sun J, Wang Q, Qiao Z, Bai DQ, Sun J, Qiao X. Effect of lipopolysaccharide (LPS) and outer membrane protein (OMP) vaccines on protection of grass carp (Ctenopharyngodon idella) against Aeromonas hydrophila. The Israeli Journal of Aquaculture - Bamidgeh. 2011; 63:1-8. http://hdl.handle.net/10524/22913
- Swain P, Behura A, Dash S, Nayak SK. Serum antibody response of Indian major carp, Labeo rohita to three species of pathogenic bacteria; Aeromonas hydrophila, Edwardsiella tarda and Pseudomonas fluorescens. Veterinary Immunology and Immunopathology. 2007; 117:137-141. https://doi.org/10.1016/j.vetimm. 2007.02.010
- Uribe C, Folch H, Enriquez R, Moran G. Innate and adaptive immunity in teleost fish: a review. Veterinarni Medicina. 2011; 56(10):486-503.
![Table 1: OD values after 28" d post vaccination (Ri and R2) as assessed by ELISA [Data (n=3) are presented as mean+standard deviation. Values with different lower case letter superscripts differ significantly (P< 0.05) between the control and different treatment within the same column. Values with different upper case letter superscripts differ significantly (P< 0.05) between in vitro humoral immunity assessment against homologous and heterologus antigen cross-response within the same vaccinated groups. ]](https://figures.academia-assets.com/82634179/table_001.jpg) ](
](