Dominant negative mutant cyclin T1 proteins that inhibit HIV transcription by forming a kinase inactive complex with Tat (original) (raw)
Abstract
Transcription of the human immunodeficiency virus type 1 (HIV) requires the interaction of the cyclin T1 (CycT1) subunit of a host cellular factor, the positive transcription elongation factor b (P-TEFb), with the viral Tat protein, at the transactivation response element (TAR) of nascent transcripts. Because of this virus-specific interaction, CycT1 may potentially serve as a target for the development of anti-HIV therapies. Here we report the development of a mutant CycT1 protein, containing three threonine-to-alanine substitutions in the linker region between two of the cyclin boxes, which displays a potent dominant negative effect on HIV transcription. Investigation into the inhibitory mechanism revealed that this mutant CycT1 interacted with Tat and the cyclin-dependent kinase 9 (Cdk9) subunit of P-TEFb, but failed to stimulate the Cdk9 kinase activity critical for elongation. This mutant CycT1 protein may represent a novel class of specific inhibitors of HIV transcription whic...
FAQs
AI
What impact do specific threonine mutations have on CycT1 functionality?add
Mutations of threonines T143, T149, and T155 in CycT1 result in a triple mutant (CycT1-280) that loses the ability to support Tat-transactivation, indicating their critical role in functionality.
How does the truncated CycT1-280 mutant affect HIV transcription?add
The CycT1-280 (T143, 149, 155A) mutant inhibits HIV transcription by approximately 85% when overexpressed alongside Tat, demonstrating its dominant negative effect.
What is the significance of CycT1's interactions with Cdk9 and Tat?add
CycT1 is essential for HIV transcription as it forms complexes with both Tat and Cdk9, which activate transcription elongation.
What methodology supports the dominance of mutant CycT1 in inhibiting transcription?add
Co-expression and immunoprecipitation assays reveal that the mutant CycT1-280 retains binding to Tat but blocks its function by preventing effective kinase activity.
When was the structural relationship of CycT1 determined?add
Previous studies clarified the crystal structure of the cyclin box region of CycT1, elucidating functional motifs critical for its interactions.
Figures (3)
Fig. 2. CycT1-280 (1143, 149, 155A) proteins associate with Cdk9 and Tat as a kinase-negative complex. (a) HA-tagged wt (lane 2) and mutant CycT1-280 (T1438, 149, 155A) proteins (lane 3) were overexpressed in 298T cells in the presence of myc- tagged Tat proteins, and immunoprecipitated from the cell using anti-HA antibodies. Tat and the endogenous Cdk9 associated with HA-CycT1 were detected by anti-myc and anti-Cdk9 antibodies, respectively. (b) The truncated version of CycT1 (1-280) does not interact with HEXIM1 in the presence of Tat. HA-tagged full-length (1-726; lane 1) and truncated (1-280) CycT1 [wt, lane 2, and CycT1-280 (1143, 149, 155A), lane 3] proteins were over- expressed in 293T cells in the presence of FLAG-tagged HEXIM1 proteins and myc-tagged Tat proteins and immunoprecipitated from the cell lysates with anti-HA antibodies. The HEXIM1 proteins associated with HA-CycT1 were detected by anti-FLAG antibod- ies. (c) Tat proteins associated with the mutant CycT1-280 (1143, 149, 155A) proteins have a lower CTD-kinase activity. Myc- epitope tagged Tat proteins were overexpressed in 298T cells in the absence (lane 1) or presence (lane 2) of CycT1-280 (1143, 149, 155A), and immunoprecipitated with anti-myc antibodies. The endogenous CycT1 and Cdk9 proteins, and HA-CycT1 (1- 280) associated with Tat were detected by Western blot analysis as indicated. The Tat-associated kinase (TAK) assays were performed as described previously (Herrmann et a/., 1998).

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (33)
- Anand, K., Schulte, A., Fujinaga, K., Scheffzek, K. & Geyer, M. (2007). Cyclin box structure of the P-TEFb subunit cyclin T1 derived from a fusion complex with EIAV tat. J Mol Biol 370, 826-836.
- Bai, J., Sui, J., Zhu, R. Y., Tallarico, A. S., Gennari, F., Zhang, D. & Marasco, W. A. (2003). Inhibition of Tat-mediated transactivation and HIV-1 replication by human anti-hCyclinT1 intrabodies. J Biol Chem 278, 1433-1442.
- Barboric, M., Yik, J. H., Czudnochowski, N., Yang, Z., Chen, R., Contreras, X., Geyer, M., Matija Peterlin, B. & Zhou, Q. (2007). Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Res 35, 2003-2012.
- Bieniasz, P. D., Grdina, T. A., Bogerd, H. P. & Cullen, B. R. (1998). Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. EMBO J 17, 7056- 7065.
- Bieniasz, P. D., Grdina, T. A., Bogerd, H. P. & Cullen, B. R. (1999). Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc Natl Acad Sci U S A 96, 7791- 7796.
- Chao, S. H. & Price, D. H. (2001). Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem 276, 31793-31799.
- Chao, S. H., Fujinaga, K., Marion, J. E., Taube, R., Sausville, E. A., Senderowicz, A. M., Peterlin, B. M. & Price, D. H. (2000). Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem 275, 28345-28348.
- Dames, S. A., Schonichen, A., Schulte, A., Barboric, M., Peterlin, B. M., Grzesiek, S. & Geyer, M. (2007). Structure of the cyclin T binding domain of Hexim1 and molecular basis for its recognition of P-TEFb. Proc Natl Acad Sci U S A 104, 14312-14317.
- Diehl, J. A. & Sherr, C. J. (1997). A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol 17, 7362-7374.
- Fujinaga, K., Taube, R., Wimmer, J., Cujec, T. P. & Peterlin, B. M. (1999). Interactions between human cyclin T, Tat, and the transactivation response element (TAR) are disrupted by a cysteine to tyrosine substitution found in mouse cyclin T. Proc Natl Acad Sci U S A 96, 1285-1290.
- Fujinaga, K., Irwin, D., Geyer, M. & Peterlin, B. M. (2002). Optimized chimeras between kinase-inactive mutant Cdk9 and truncated cyclin T1 proteins efficiently inhibit Tat transactivation and human immunodeficiency virus gene expression. J Virol 76, 10873-10881.
- Garber, M. E., Wei, P. & Jones, K. A. (1998a). HIV-1 Tat interacts with cyclin T1 to direct the P-TEFb CTD kinase complex to TAR RNA. Cold Spring Harb Symp Quant Biol 63, 371-380.
- Garber, M. E., Wei, P., KewalRamani, V. N., Mayall, T. P., Herrmann, C. H., Rice, A. P., Littman, D. R. & Jones, K. A. (1998b). The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev 12, 3512-3527.
- Heredia, A., Davis, C., Bamba, D., Le, N., Gwarzo, M. Y., Sadowska, M., Gallo, R. C. & Redfield, R. R. (2005). Indirubin-39-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS 19, 2087-2095.
- Herrmann, C. H., Carroll, R. G., Wei, P., Jones, K. A. & Rice, A. P. (1998). Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J Virol 72, 9881-9888.
- Hwang, S., Tamilarasu, N., Kibler, K., Cao, H., Ali, A., Ping, Y. H., Jeang, K. T. & Rana, T. M. (2003). Discovery of a small molecule Tat- trans-activation-responsive RNA antagonist that potently inhibits human immunodeficiency virus-1 replication. J Biol Chem 278, 39092-39103.
- Ivanov, D., Kwak, Y. T., Nee, E., Guo, J., Garcia-Martinez, L. F. & Gaynor, R. B. (1999). Cyclin T1 domains involved in complex formation with Tat and TAR RNA are critical for tat-activation. J Mol Biol 288, 41-56.
- Karn, J. (1999). Tackling Tat. J Mol Biol 293, 235-254.
- Kim, Y. K., Bourgeois, C. F., Isel, C., Churcher, M. J. & Karn, J. (2002).
- Kim, Y. K., Bourgeois, C. F., Pearson, R., Tyagi, M., West, M. J., Wong, J., Wu, S. Y., Chiang, C. M. & Karn, J. (2006). Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J 25, 3596-3604.
- Lind, K. E., Du, Z., Fujinaga, K., Peterlin, B. M. & James, T. L. (2002).
- Structure-based computational database screening, in vitro assay, and NMR assessment of compounds that target TAR RNA. Chem Biol 9, 185-193.
- Mancebo, H. S., Lee, G., Flygare, J., Tomassini, J., Luu, P., Zhu, Y., Peng, J., Blau, C., Hazuda, D. & other authors (1997). P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev 11, 2633-2644.
- Michels, A. A., Nguyen, V. T., Fraldi, A., Labas, V., Edwards, M., Bonnet, F., Lania, L. & Bensaude, O. (2003). MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol Cell Biol 23, 4859-4869.
- Mischiati, C., Jeang, K. T., Feriotto, G., Breda, L., Borgatti, M., Bianchi, N. & Gambari, R. (2001). Aromatic polyamidines inhibiting the Tat-induced HIV-1 transcription recognize structured TAR-RNA. Antisense Nucleic Acid Drug Dev 11, 209-217.
- Napolitano, G., Mazzocco, A., Fraldi, A., Majello, B. & Lania, L. (2003). Functional inactivation of Cdk9 through oligomerization chain reaction. Oncogene 22, 4882-4888.
- Nguyen, V. T., Kiss, T., Michels, A. A. & Bensaude, O. (2001). 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322-325.
- Okamoto, H., Cujec, T. P., Okamoto, M., Peterlin, B. M., Baba, M. & Okamoto, T. (2000). Inhibition of the RNA-dependent transactiva- tion and replication of human immunodeficiency virus type 1 by a fluoroquinoline derivative K-37. Virology 272, 402-408.
- Peterlin, B. M. & Price, D. H. (2006). Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23, 297-305.
- Richter, S. N. & Palu, G. (2006). Inhibitors of HIV-1 Tat-mediated transactivation. Curr Med Chem 13, 1305-1315.
- Schulte, A., Czudnochowski, N., Barboric, M., Schonichen, A., Blazek, D., Peterlin, B. M. & Geyer, M. (2005). Identification of a cyclin T-binding domain in Hexim1 and biochemical analysis of its binding competition with HIV-1 Tat. J Biol Chem 280, 24968-24977.
- Taube, R., Fujinaga, K., Wimmer, J., Barboric, M. & Peterlin, B. M. (1999). Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology 264, 245-253.
- Yang, Z., Zhu, Q., Luo, K. & Zhou, Q. (2001). The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317-322.
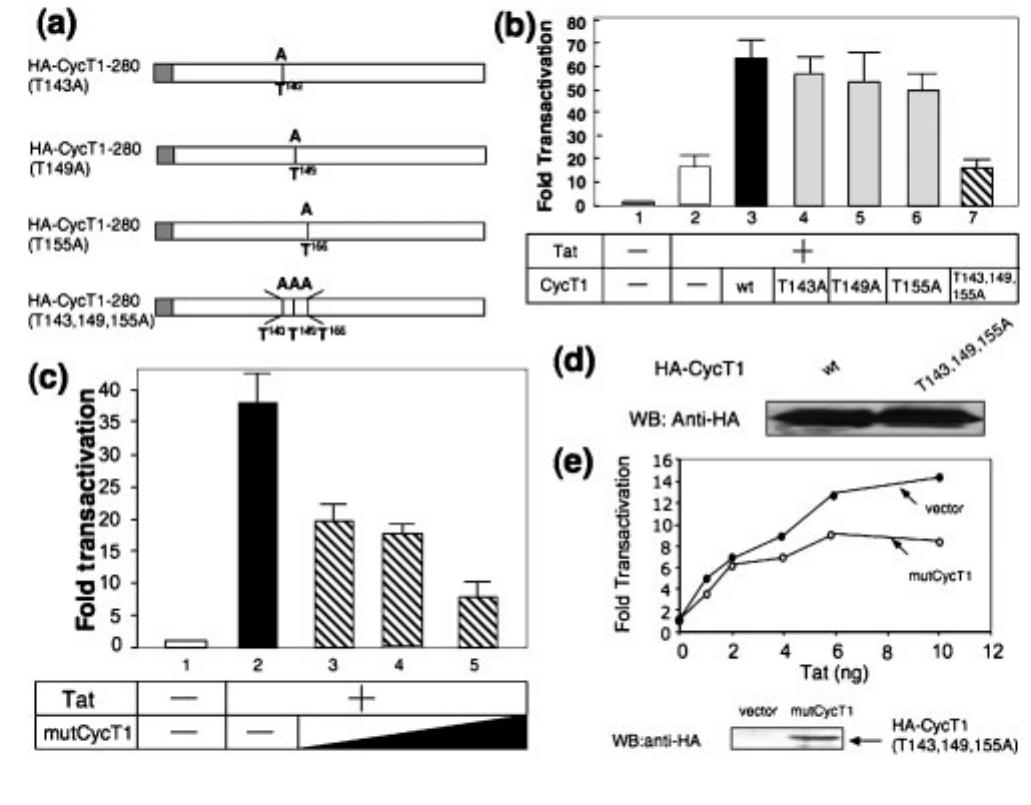
![Fig. 2. CycT1-280 (1143, 149, 155A) proteins associate with Cdk9 and Tat as a kinase-negative complex. (a) HA-tagged wt (lane 2) and mutant CycT1-280 (T1438, 149, 155A) proteins (lane 3) were overexpressed in 298T cells in the presence of myc- tagged Tat proteins, and immunoprecipitated from the cell using anti-HA antibodies. Tat and the endogenous Cdk9 associated with HA-CycT1 were detected by anti-myc and anti-Cdk9 antibodies, respectively. (b) The truncated version of CycT1 (1-280) does not interact with HEXIM1 in the presence of Tat. HA-tagged full-length (1-726; lane 1) and truncated (1-280) CycT1 [wt, lane 2, and CycT1-280 (1143, 149, 155A), lane 3] proteins were over- expressed in 293T cells in the presence of FLAG-tagged HEXIM1 proteins and myc-tagged Tat proteins and immunoprecipitated from the cell lysates with anti-HA antibodies. The HEXIM1 proteins associated with HA-CycT1 were detected by anti-FLAG antibod- ies. (c) Tat proteins associated with the mutant CycT1-280 (1143, 149, 155A) proteins have a lower CTD-kinase activity. Myc- epitope tagged Tat proteins were overexpressed in 298T cells in the absence (lane 1) or presence (lane 2) of CycT1-280 (1143, 149, 155A), and immunoprecipitated with anti-myc antibodies. The endogenous CycT1 and Cdk9 proteins, and HA-CycT1 (1- 280) associated with Tat were detected by Western blot analysis as indicated. The Tat-associated kinase (TAK) assays were performed as described previously (Herrmann et a/., 1998).](https://figures.academia-assets.com/84256724/figure_002.jpg) ](
](