Clays from the Bay of Naples (Italy): New insight on ancient and traditional ceramics (original) (raw)
Abstract
The features of two clayey raw materials from the Bay of Naples and their fired products were investigated via minero-petrographic and physical techniques. Clay preparation and firing dynamics were performed following a process similar to that performed by ancient and traditional potters. A high-CaO marine clay from Ischia was mixed with different amounts of volcanic temper in order to replicate most common ware. These mixtures show a fair mechanical resistance starting from relatively low firing temperatures (>850 • C). The addition of temper resulted in different technological characteristics. A low-CaO weathered pyroclastics from the Sorrento Peninsula was prepared to simulate heat resistant and refractory ceramics. Fired products are characterised by a less resistant ceramic body up to 1000 • C compared to Ischia ceramics. Despite worse strength these ceramics show a porous structure, yielding better refractory performances.
Figures (17)
Fig. 1. Grain size distribution of IS and SO clayey raw materials and AQM temper.
Grain size of raw materials (clays and temper) and Atterberg limits of raw clays: wi, liquid limit; wp, plastic limit; ws, Atterberg shrinkage limit; PI: plasticity index. Organic matter content is also reported.
Chemical analysis (XRF) of major oxides (wt.%, recalculated to 100% on a LOI-free basis), trace elements (ppm) and LOI (loss on ignition, wt.%) of raw clays, « the 950°C fired ceramics, and of the temper. Abbreviation: <LLD, less than the lower limit of detection of the instrument.
Mineralogical composition (XRPD) of raw materials (clay and temper) and fired ceramics. XXXX, very abundant; XXX, abundant; XX, frequent; X, scarce. \ bbreviations: Ill-Sm, illite-smectite mixed layer; Kao, kaolinite; Chl, chlorite; dHall, dehydrated halloysite; Anl, analcime. Colour of samples from Munsell Soil olour Chart was also reported.
Fig. 2. XRPD patterns of raw and fired IS (a) and SO (b) samples. Abbreviations: Qtz, quartz; Fs, feldspar; Cc, calcite; Dol, dolomite; Px, pyroxene; Mel, melilite; Hem, hematite; Mul, mullite; Ill, illite; Sm, smectite; Kao, kaolinite; Chl, chlorite; dHall, dehydrated halloysite.
Fig. 3. Backscattered electrons SEM images of: (a) IS 850. Newly formed melilite (Mel) rim between carbonate and clay matrix. (b) IS-C 800. Thin reaction rims of clinopyroxene (Cpx; Al-rich diopside) composition. (c) IS 1100. Large reaction rim with fingered geometry of wollastonite (Wo) around a pre-existing carbonate in contact with quartz (Qz) and glassy amorphous phase (am). (d) IS-C 900. Newly formed cuspidine (Csp) on the edge of an albite (Ab) crystal in contact with carbonate mass and fluorite (Fl). (e) IS-C 1100. Newly formed cuspidine (Csp) and clinopyroxene (Cpx). (f) Backscattered electrons FESEM images of sample SO 1100. Iron oxides particles (white dots) in amorphous phase. Among other Ca-silicates, we must point out the occur- rence of newly formed cuspidine [CaqSi207(F,OH)2] observed at SEM-EDS between 900 and 1100°C (Table 5). We detected cuspidine at the edges of silicates (feldspars and quartz) in ferroan aluminian diopside.*° The initial development of thin reaction rims tending to this composition was detected via SEM-EDS already starting from 800°C (Fig. 3b). At this T the presence of pyroxene was also noticed in the XRPD pattern, then a slight rise of its reflection was recorded starting from 850°C, whereas a tangible increase of pyroxene amount was observed at 1100°C. This might also be due to the wollastonite formation by reaction between pre-existing gehlenite and quartz
Representative EDS-analyses (wt.%) and cation proportion (a.p.f.u.) of melilites in IS ceramics. Table 4 Table 5 Representative EDS-analyses (wt.%) and cation proportion (a.p.f.u.) of cuspidine, clinopyroxene, and wollastonite in IS ceramics.
Modal analysis of the temper (AQM) and of representative ceramics fired at 700, 900, and 1100 °C. Bold fonts indicate the contribution of AQM in tempered ceramics Abbreviations: A-fs, alkali feldspar; Pl, plagioclase; Cpx, clinopyroxene; Pum, pumices; Sc, volcanic scoriae; VL, volcanic lithics; Ol, olivine; Cb, carbonates; ARF Argillaceous Rock Fragments. Mullite was detected in the SO ceramics at maximum fir- ing T (1100°C) after the decomposition of phyllosilicates.** Nevertheless, neither hematite nor mullite could be analysed at SEM-EDS because of their very small size. In addition to hematite and mullite, these ceramics did not show any other pyrometamorphic transformation. contact with fluorite-bearing carbonate masses (Fig. 3d), likely formed according to the following reaction*?:
Fig. 4. PLM images of: (a) IS 700. Natural inclusions of IS ceramics (crossed polars). (b) IS 800. Sporadic large volcanic grain in non-tempered ceramics (crossec polars). Backscattered electrons FESEM images of (c) IS-C 700, exfoliated phyllosilicate for dehydroxylation, and (d)IS-C 700, carbonate microfossil. (e) IS 1100 Decomposed calcite (crossed polars). (f) AQM. Sand used as temper with alkali feldspar, clinopyroxene, pumices, and scoriae (parallel polars). (g) IS-C 700. Tempet grains (pumices and scoriae) in the ceramic paste (parallel polars). (h) SO dry. Leucite-bearing scoriae (crossed polars). (i) SO 700. Garnet and alkali feldspar (paralle: polars).
Fig. 5. SEM images of freshly fractured samples: (a) IS-C 1000. Discontinuities at temper/matrix interface. (b) IS 800. Poorly sintered structure. (c) IS-A 1100 Highly vitrified structure with rounded isolated pores. (d) SO 850. Poorly sintered structure. (e) SO 1000. Vitrified structure. (f) SO 1100. Continuous vitrificatior with non-connected pores.
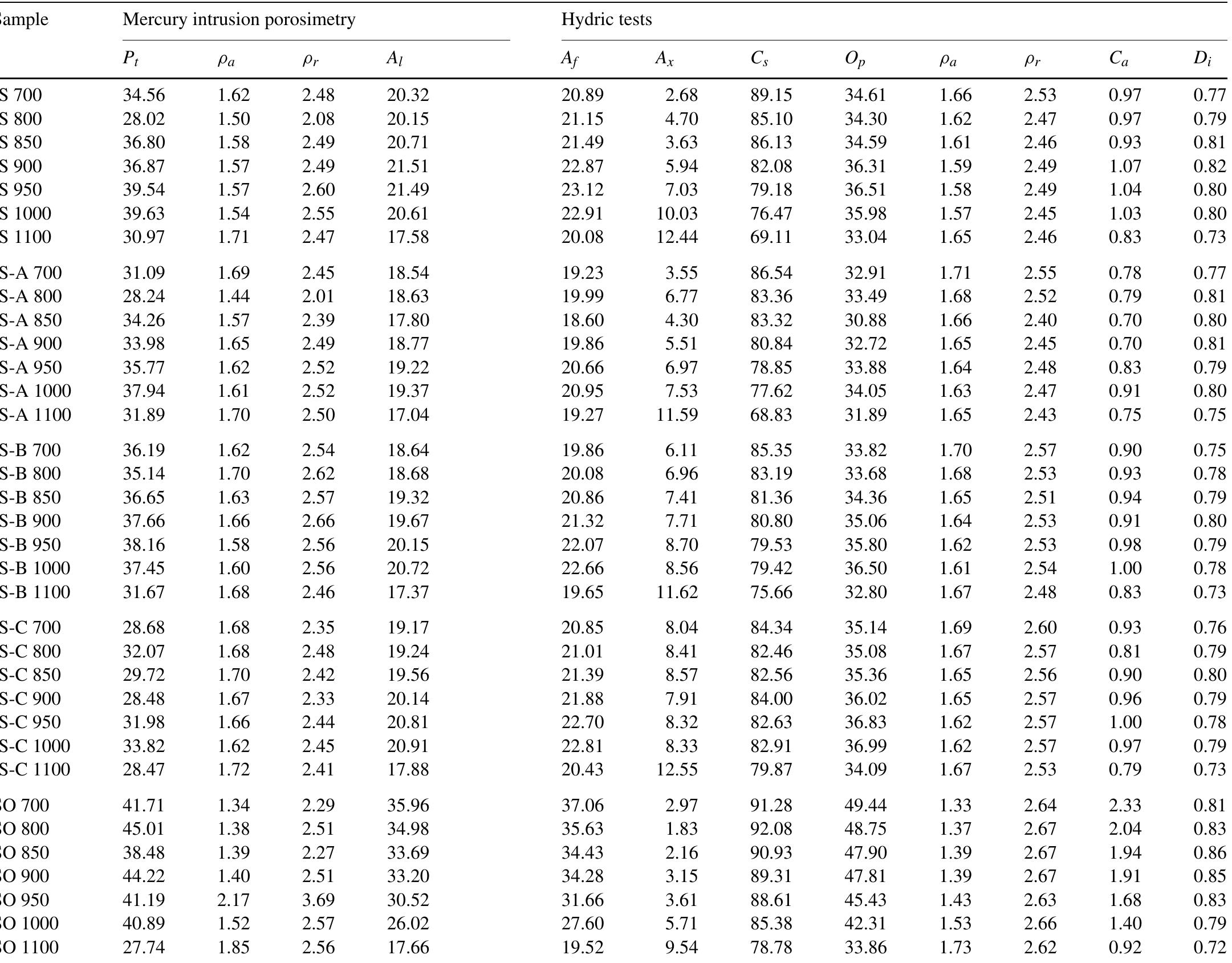
P,, total porosity (%); og, apparent density (gcm~>); p,, real density (gcm74) values of ceramics acquired via mercury intrusion porosimetry (MIP) along with hydric tests values of ceramics: A), free water adsorption (%); Ay, forced water adsorption (%); Ax, degree of pore interconnectivity (%); C;, saturation coefficient (%); Op, open porosity (%); Oa, apparent density (g cm~); p;, real density (gcm~+); C,, absorption coefficient; D;, drying index.
Fig. 6. (a) Mass change of fired samples due to free water absorption, desorption during hydric tests. (b) MIP pore size distribution curves of fired samples. Lo; differential intruded volume (ml/g) vs. pore radius (wm).
Fig. 7. Diagrams showing the variation of (a) free water absorption (A;) and of (b) open porosity (O,) for IS ceramics in relation with the temper percentage.
Ultrasound wave velocity V, (m/s) and V; (m/s) of ceramics with elastic moduli: G, shear modulus (GPa); E, Young’s modulus (GPa); K, bulk modulus (GPa).
Fig. 8. Diagrams showing the variation of (a) ultrasound velocity (V,) and (b) Young’s modulus (£) vs. firing T
Fig. 9. Representative IR thermographic images representing the height of the isotherm after 20 min of heating in (a) high-CaO ceramics fired at 700 (IS 700) and 1100 C CIS 1100), and (b) high-CaO (IS 900) and low-CaO (SO 900) ceramics fired at the same T (900°C).

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (68)
- De Bonis A, Grifa C, Cultrone G, De Vita P, Langella A, Morra V. Raw materials for archaeological pottery from the Campania Region of Italy: a petrophysical characterization. Geoarchaeology 2013;28(5):478-503.
- Morel JP. La ceramica campana A nell'economia della Campania. In: Napoli Antica, Catalogo della Mostra. Napoli (Italy).; 1985. p. 372-8.
- Morel JP. Remarques sur l'art et l'artisanat de Naples antique. In: Neapolis. Atti del XXV CMGr, Taranto 3-7 ottobre 1985, Taranto (Italy).; 1986. p. 305-56.
- Grifa C, Morra V, Langella A, Munzi P. Byzantine ceramic productions from Cuma (Campi Flegrei, Napoli). Archaeometry 2009;51(1):75-94.
- Morra V, De Bonis A, Grifa C, Langella A, Cavassa L, Piovesan R. Minero-petrographic study of cooking ware and Pompeian Red Ware (Rosso Pompeiano) from Cuma (Southern Italy). Archaeometry 2013;55(5):852-79.
- Grifa C, De Bonis A, Langella A, Mercurio M, Soricelli G, Morra V. A Late Roman ceramic production from Pompeii. J Archaeol Sci 2013;40(2):810-26.
- Giampaola D, Febbraro S, De Bonis A, Guarino V, Morra V, et al. The pottery workshop area at Piazza Nicola Amore, Naples. Black-glaze and common ware production: archaeology and archaeometry. In: Greco G, Cicala L, editors. Archaeometry. Comparing Experiences, Quaderni del Centro Studi Magna Grecia, 19. Naus Editoria: Università degli Studi di Napoli, Federico II. Pozzuoli (NA); 2014 [in press].
- Peters T, Iberg R. Mineralogical changes during firing of calcium-rich brick clays. Am Ceram Soc Bull 1978;57:503-9.
- Maniatis Y, Tite MS. Technological examination of Neolithic-Bronze Age pottery from central and southeast Europe and from the Near East. J Archaeol Sci 1981;8:59-76.
- Cultrone G, Sebastián E, Elert K, de la Torre MJ, Cazalla O, Rodríguez- Navarro C. Influence of mineralogy and firing temperature on the porosity of bricks. J Eur Ceram Soc 2004;24:547-64.
- Grifa C, Cultrone G, Langella A, Mercurio M, De Bonis A, Sebastián E, et al. Ceramic replicas of archaeological artefacts in Benevento area (Italy): petrophysical changes induced by different proportions of clays and temper. Appl Clay Sci 2009;46(3):231-40.
- Brown RJ, Orsi G, de Vita S. New insights into late Pleistocene explo- sive volcanic activity and caldera formation on Ischia (southern Italy). Bull Volcanol 2008;70(5):583-603.
- Buchner G, Rittmann A. Origine e passato dell'isola d'Ischia. Napoli (Italy): Macchiaroli; 1948. p. 77.
- Buchner G. I giacimenti di argilla dell'isola d'Ischia e l'industria figulina locale in età recente. In: Quaderno del Centro studi per la storia della ceramica meridionale. Bari (Italy).; 1994. p. 17-45.
- De Bonis A, Grifa C, Langella A, Mercurio M, Perrone ML, Morra V. Archaeometric study of Roman pottery from caudium area (Southern Italy). Period Miner 2010;79:73-89.
- Guarino V, De Bonis A, Grifa C, Langella A, Morra V, Pedroni L. Archaeometric study on terra sigillata from Cales (Italy). Period Miner 2011;80:455-70.
- Grifa C, Langella A, Morra V, Soricelli G. Pantellerian ware from Miseno (Campi Flegrei, Napoli). Period Miner 2005;74(1):69-86.
- Capriglione C, De Bonis A, De Tommaso G, Guarino V, Iuliano M, Marino D, et al. Grandi dolii protostorici d'impasto dalla Calabria centromerid- ionale. Contributo allo studio cronotipologico, tecnologico e funzionale. Rivista di Scienze Preistoriche 2012;62:331-62.
- Tite MS, Kilikoglou V, Vekinis G. Strength, toughness and thermal shock resistance of ancient ceramics, and their influence on technological choice. Archaeometry 2001;43(3):301-24.
- ASTM Test designation D2217. Standard practice for wet preparation of soil samples for particle-size analysis and determination of soil constants, vol. 04.08. Philadelphia, USA: ASTM; 1985.
- ASTM Test designation D422. Standard test method for particle-size anal- ysis of soils, vol. 04.08. Philadelphia, USA: ASTM; 1972.
- ASTM Test designation D421. Standard practice for dry preparation of soil samples for particle-size analysis and determination of soil constants, vol. 04.08. Philadelphia, USA: ASTM; 1985.
- ASTM Test designation D4318. Standard test methods for liquid limit, plas- tic limit, and plasticity index of soils, vol. 04.08. Philadelphia, USA: ASTM; 1984.
- ASTM Test designation D4943. Standard test method for shrinkage factors of soils by the wax method, vol. 04.08. Philadelphia, USA: ASTM; 1989.
- BSI British Standard BS 1377-2. Methods of test for soils for civil engi- neering purposes. Classification tests. London, UK: British Standards Institution; 1990.
- ASTM Test designation D2974. Standard test methods for moisture, ash, and organic. Matter of peat and other organic soils, vol. 04.08. Philadelphia, USA: ASTM; 2000.
- ASTM Test designation C24 -09. Standard test method for pyrometric cone equivalent (PCE) of fireclay and high alumina refractory materials, vol. 15.01. Philadelphia, USA: ASTM; 2013.
- Cultrone G, Rodríguez-Navarro C, Sebastián E, Cazalla O, de la Torre MJ. Carbonate and silicate phase reactions during ceramic firing. Eur J Miner 2001;13:621-34.
- Bish DL, Reynolds Jr RC. Modern powder diffraction. In: Bish DL, Post JE, editors. Reviews in mineralogy, vol. 20. Washington, DC: Mineralogical Society of America; 1989. p. 73-99.
- Moore DM, Reynolds RC. X-ray diffraction and the identification and anal- ysis of clay minerals. New York: Oxford University Press; 1997. p. 378.
- Melluso L, Morra V, Brotzu P, Tommasini S, Renna MR, Dun- can RA, et al. Geochronology and petrogenesis of the cretaceous Antampombato-Ambatovy complex and associated dyke swarm, Madagas- car. J Petrol 2005;46:1963-96.
- NORMAL 7/81. Assorbimento dell'acqua per immersione totale. Capacità di imbibizione. Rome, Italy: CNR-ICR; 1981.
- RILEM TC 25-PEM. Recommended tests to measure the deterioration of stone and to assess the effectiveness of treatment methods. Com- mission 25-PEM: Protection et Erosion des Monuments. Mater Struct 1980;13(75):175-253.
- NORMAL 29/88. Misura dell'indice di asciugamento (Drying Index). Rome, Italy: CNR-ICR; 1988.
- ASTM Test designation D2845. Standard method for laboratory determina- tion of pulse velocities and ultrasonic elastic constants of rock, vol. 04.08. Philadelphia, USA: ASTM; 1983.
- Cultrone G, Sebastián E, Cazalla O, Nechar M, Romero R, Bagur MG. Ultrasound and mechanical tests combined with ANOVA to evaluate brick quality. Ceram Int 2001;27:401-6.
- Prassianakis IN, Prassianakis NI. Ultrasonic testing of non-metallic materi- als: concrete and marble. Theor Appl Fract Mech 2004;42:191-8.
- Morra V, Calcaterra D, Cappelletti P, Colella A, Fedele L, de Gennaro R, et al. Urban geology: relationships between geological setting and archi- tectural heritage of the Neapolitan area. In: Beltrando M, Peccerillo A, Mattei M, Conticelli S, Doglioni C, editors. The Geology of Italy: tectonics and life along plate margins, J Virt Explorer, vol. 36, paper 27, Electronic Edition. 2010., http://dx.doi.org/10.3809/jvirtex.2010.00261, available at: http://virtualexplorer.com.au/journal/2010/36
- Adamo P, Violante P, Wilson MJ. Tabular and spheroidal halloysite in pyro- clastic deposits in the area of the Roccamonfina volcano (Southern Italy). Geoderma 2001;99:295-316.
- de' Gennaro M, Cappelletti P, Langella A, Perrotta A, Scarpati C. Genesis of zeolites in the Neapolitan Yellow Tuff: geological, volcanological and mineralogical evidence. Contrib Miner Petrol 2000;139:17-35.
- Boynton RS. Chemistry and technology of lime and limestone. 2nd ed. New York, USA: John Wiley & Sons Inc; 1980. p. 578.
- Dondi M, Ercolani G, Guarini G, Marsigli M, Venturi I. Evoluzione della microstruttura durante la cottura rapida di impasti per piastrelle porose. Ceramurgia 1995;25(6):301-14.
- Maritan L, Nodari L, Mazzoli C, Milano A, Russo U. Influence of firing on ceramic products: experimental study on clay rich in organic matter. Appl Clay Sci 2006;31:1-15.
- Dondi M, Ercolani G, Fabbri B, Marsigli M. Chemical composition of melilite formed during the firing of carbonate-rich and iron-containing ceramic bodies. J Am Ceram Soc 1999;82(2):465-8.
- Rathossi C, Pontikes Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments. Part I: reaction paths, crystalline phases, microstructure and colour. J Eur Ceram Soc 2010;30:1841-51.
- Rathossi C, Pontikes Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments: part II. Chemistry of pyrometamorphic minerals and comparison with ancient ceramics. J Eur Ceram Soc 2010;30:1853-66.
- Dondi M, Ercolani G, Fabbri B, Marsigli M. An approach to the chemistry of pyroxenes formed during the firing of Ca-rich silicate ceramics. Clay Miner 1998;33:443-52.
- Grapes R. Pyrometamorphism. New York, USA: Springer-Verlag LLC; 2006. p. 275.
- Fukuyama H, Tabata H, Nagata K. Determination of Gibbs energy of formation of cuspidine (3CaO• • •2SiO 2 • • •CaF 2 ) from the electromotive force method using CaF 2 as the solid electrolyte. Metall Mater Trans B 2003;34(3):307-11.
- García-Ten J, Monfort E, Gomez P, Gomar S. Influence of calcite content on fluorine compound emissions during ceramic tile firing. J Ceram Process Res 2006;7:75-82.
- Nodari L, Marcuz E, Maritan L, Mazzoli C, Russo U. Hematite nucleation and growth in the firing of carbonate-rich clay for pottery production. J Eur Ceram Soc 2007;27:4665-73.
- Molera J, Pradell T, Vendrell-Saz M. The colours of Ca-rich ceramic paste: origin and characterization. Appl Clay Sci 1998;13: 187-202.
- Rodríguez-Navarro C, Cultrone G, Sánchez-Navas A, Sebastian E. TEM study of mullite growth after muscovite breakdown. Am Miner 2003;88:713-24.
- Cerdeño del Castillo J, Díaz Rubio R, Obis Sánchez J, Pérez Lorenzo A, Velasco Vélez J. Manual de patologías de las piezas cerámicas para la construcción. Toledo, Spain: Aitemin; 2000. p. 118.
- Barra D, Cinque A, Italiano A, Scorziello R. Il Pleistocene superiore marino di Ischia: paleoecologia e rapporti con l'evoluzione tettonica recente. Studi Geologici Camerti 1992;1(special issue):231-43.
- Joron JL, Métrich N, Rosi M, Santacroce R, Sbrana A. Chemistry and pet- rography. In: Santacroce R, editor. Somma-Vesuvius C.N.R. Quaderni de La Ricerca Scientifica, 114, Rome Consiglio Nazionale delle Ricerche. 1987. p. 105-74.
- Laird RT, Worcester M. The inhibiting of lime blowing. Br Ceram Trans J 1956;55:545-63.
- Kilikoglou V, Vekinis G, Maniatis Y, Day PM. Mechanical performance of quartz-tempered ceramics: part I, strength and toughness. Archaeometry 1998;40:261-79.
- Kornilov AV. Reasons for the different effects of calcareous clays on strength properties of ceramics. Glass Ceram 2005;62(11-12): 391-3.
- Cultrone G, Sebastián E, de la Torre MJ. Mineralogical and physical behaviour of solid bricks with additives. Constr Build Mater 2005;19: 39-48.
- Chen GH. Sintering, crystallization, and properties of CaO doped cordierite- based glass-ceramics. J Alloys Compd 2008;455:298-302.
- Lassinantti Gualtieri M, Gualtieri AF, Gagliardi S, Ruffini P, Ferrari R, Hanuskova M. Thermal conductivity of fired clays: effect of min- eralogical and physical properties of the raw materials. Appl Clay Sci 2010;49(3):269-75.
- Erker A. The thermal conductivity of the brick ceramic body (part 2). Ziegelindustrie International 2002;55(11):32-7.
- Hossain KMA, Lachemi M. Thermal conductivity and acoustic per- formance of volcanic pumice based composites. Mater Sci Forum 2005;480-481:611-6.
- Dondi M, Marsigli M, Venturi I. Microstructure and mechanical properties of clay bricks: comparison between fast firing and traditional firing. Br Ceram Trans 1999;98:12-8.
- Papachristodoulou C, Gravani K, Oikonomou A, Ioannides K. On the provenance and manufacture of red-slipped fine ware from ancient Cas- sope (NW Greece): evidence by X-ray analytical methods. J Archaeol Sci 2010;37:2146-54.
- Carter CB, Norton MG. Ceramic materials: science and engineering. New York, USA: Springer-Verlag LLC; 2007. p. 716.
- Hein A, Müller NS, Day PM, Kilikoglou V. Thermal conductivity of archae- ological ceramics: the effect of inclusions, porosity and firing temperature. Thermochim Acta 2008;480:35-42.





![Fig. 3. Backscattered electrons SEM images of: (a) IS 850. Newly formed melilite (Mel) rim between carbonate and clay matrix. (b) IS-C 800. Thin reaction rims of clinopyroxene (Cpx; Al-rich diopside) composition. (c) IS 1100. Large reaction rim with fingered geometry of wollastonite (Wo) around a pre-existing carbonate in contact with quartz (Qz) and glassy amorphous phase (am). (d) IS-C 900. Newly formed cuspidine (Csp) on the edge of an albite (Ab) crystal in contact with carbonate mass and fluorite (Fl). (e) IS-C 1100. Newly formed cuspidine (Csp) and clinopyroxene (Cpx). (f) Backscattered electrons FESEM images of sample SO 1100. Iron oxides particles (white dots) in amorphous phase. Among other Ca-silicates, we must point out the occur- rence of newly formed cuspidine [CaqSi207(F,OH)2] observed at SEM-EDS between 900 and 1100°C (Table 5). We detected cuspidine at the edges of silicates (feldspars and quartz) in ferroan aluminian diopside.*° The initial development of thin reaction rims tending to this composition was detected via SEM-EDS already starting from 800°C (Fig. 3b). At this T the presence of pyroxene was also noticed in the XRPD pattern, then a slight rise of its reflection was recorded starting from 850°C, whereas a tangible increase of pyroxene amount was observed at 1100°C. This might also be due to the wollastonite formation by reaction between pre-existing gehlenite and quartz](https://figures.academia-assets.com/87700895/figure_003.jpg) ](
](