Digital Morphometrics: A Tool for Leaf Morpho- Taxonomical Studies (original) (raw)
Abstract
Leaves are most important part of the plant and can be used for the identification of a taxon. An appropriate understanding of leaf development in terms of shape and responsible abiotic factors is necessary for improvement in plant. Leaf shape variation could be evaluated successfully, and the symmetrical and asymmetrical elements of the overall shape variation could be detected. The aim of the present study was to establish a quantitative analysis method of leaf shape by elliptic Fourier descriptors and principal component analysis (EF-PCA). EF-PCA describes an overall shape mathematically by transforming coordinate information concerning its contours into elliptic Fourier descriptors (EFDs) and summarizing the EFDs by principal component analysis. We can be able to extract six variables by using leaf specimen images from field and herbarium specimens. In the present study, total leaf area with respect to notch area is more variable within species. Within a species the major source...
FAQs
AI
What insights does geometric morphometrics provide in leaf shape analysis?add
The study demonstrates that geometric morphometrics effectively quantifies leaf shape variations, revealing that the first three principal components account for over 80% of total variance in several Convolvulaceae species.
How did leaf plasticity correlate with environmental conditions according to the study?add
The findings suggest that temperature fluctuations can increase leaf shape plasticity, with distinct morphological characteristics observed in leaves from colder climates.
What methodology was used for leaf shape data analysis in this research?add
Elliptic Fourier Analysis and Principal Component Analysis were employed to analyze 1440 leaf samples, allowing for a quantitative comparison of leaf outlines.
When did modern morphometric methods start gaining traction in plant taxonomy?add
In the past decade, there has been growing interest in using modern geometric morphometrics for studying plant leaf shapes, despite traditional methods remaining prevalent.
What role did environmental variability play in leaf form differentiation?add
The study indicates that morphological changes in leaves reflect environmental variability, illustrating how these factors drive phenotypic plasticity within Convolvulaceae species.
Figures (6)
Ten Commonly growing species belonging to four genera of family Convolvulaceae from Vadodara, Gujarat, India were selected for the above study. Identification of selected taxa was done through routine observation of reproductive structures and from available literature (Hooker, 1882; Cooke, 1958; Shah, 1978). Leaf development was tracked from initiation to maturity in order to identify how developmental trajectories diverge and what is conserved across species. Undamaged leaves of all the species from the initial stage of growth to the mature ones were collected and then dried in different stages of growth at different time. Leaves were kept into paper to blot dry and also to remain flat for further analysis. Then leaves were arranged from initial stage to mature one according to their size and shape. Freshly collected leaf specimens were placed on paper and color image of leaf outline of abaxial surface were captured with digital camera. The voucher specimens were deposited in BARO herbarium, department of Botany, The M S University of Baroda, Vadodara. (BARO/KO 21, 34, 41, 52, 61, 71, 79, 86, 91, 96) Figure 1: Arrangement of Leaves for Processing (Black square is scale of 30mm x 30mm)
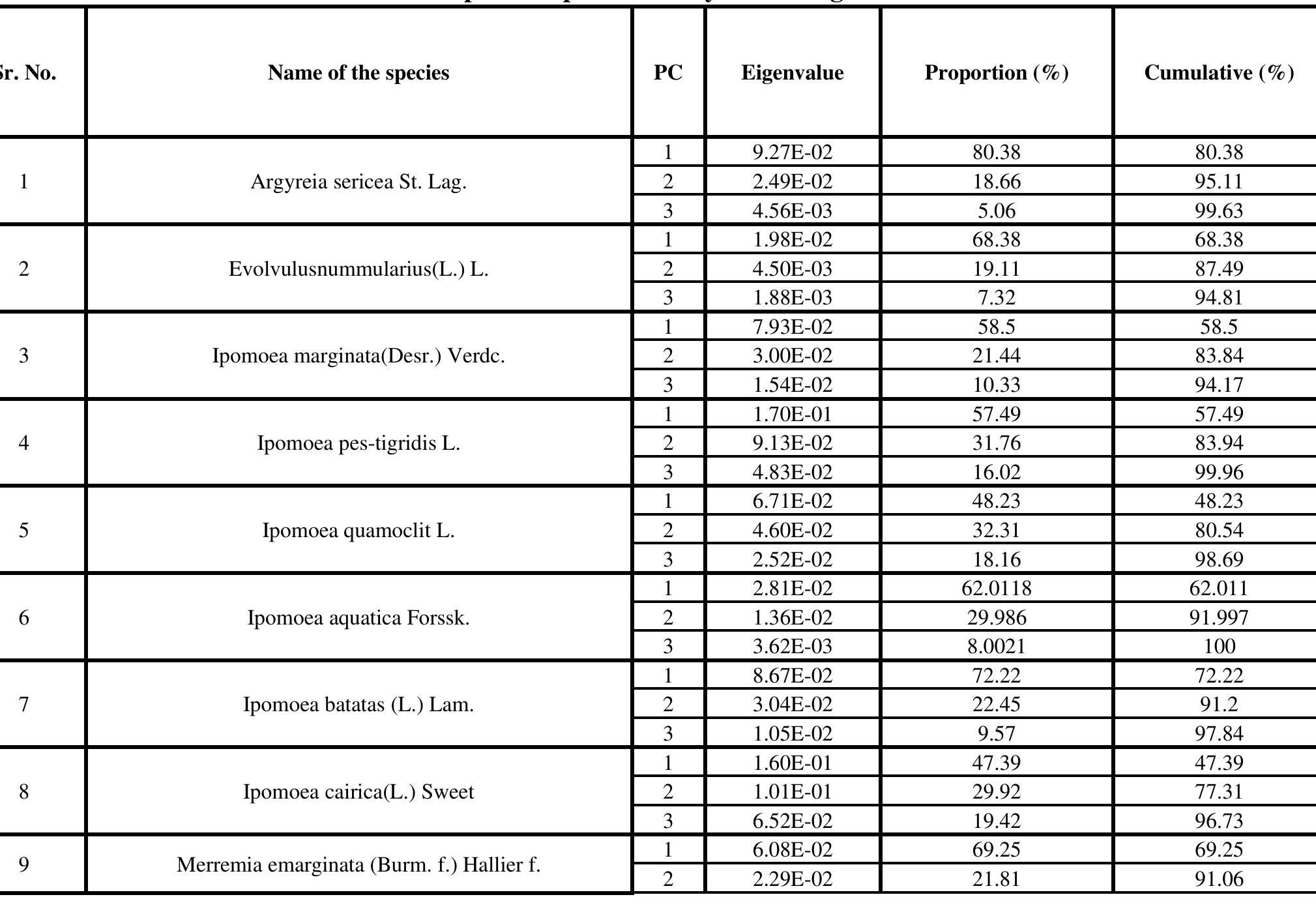
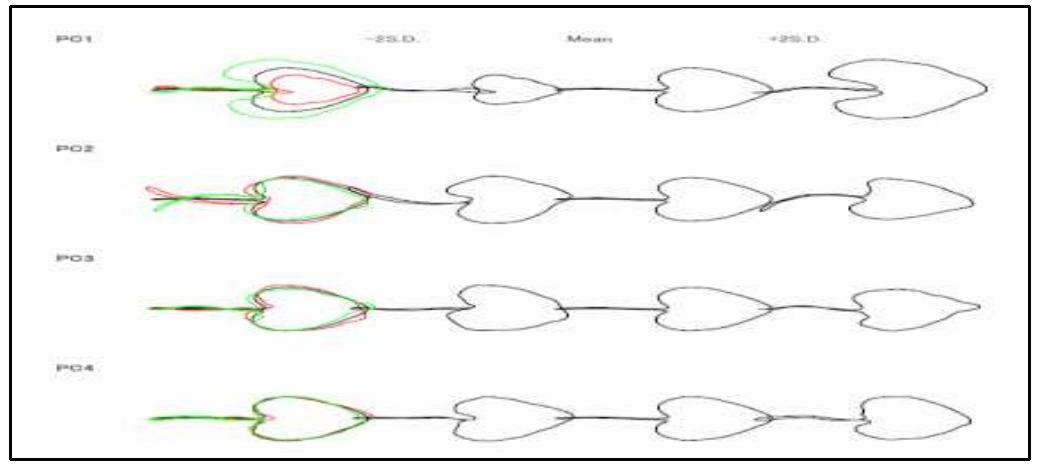
The study reveals that among the first five significant principal components scores of the coefficient of leaf mean shape, the major proportion of total variation was found in the first three principal components only. In terms of shape variables, the first three principal components accounted for over 80% of the total variance and described distinct andeasily recognized trends of leaf outline shape change. The next 3 principal components expressed ambiguous shape variations, and which were not easily figure out visually. The variations refer for the surrounding of the notch separating the posterior lobes in the base of the leaves and the correlation of leaf length to width respectively. Shape variations of the first three PCs of each species are described in Table | and also can be seen in Figure 2. Table 1: Principal Component Analysis and Eigen value matrix
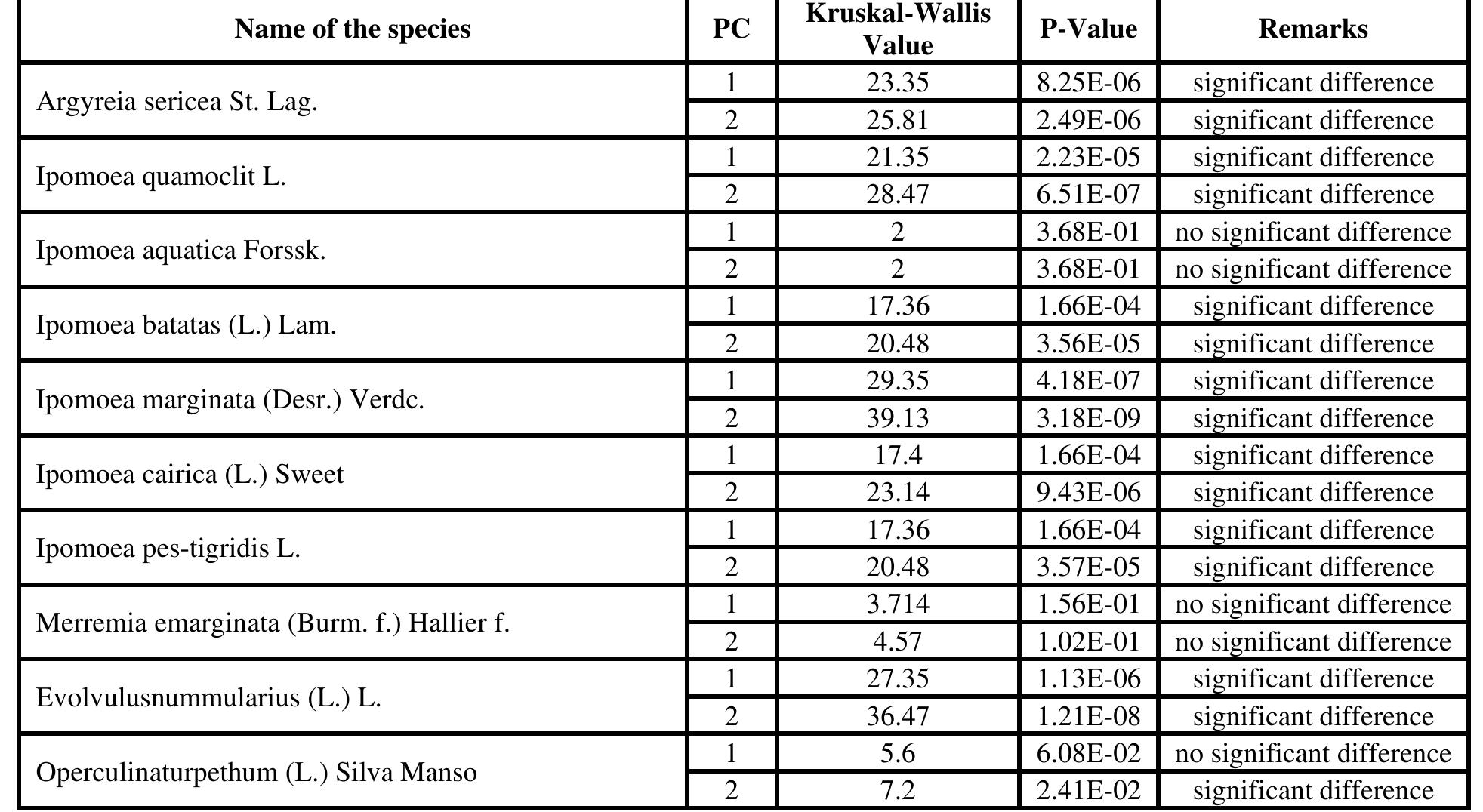
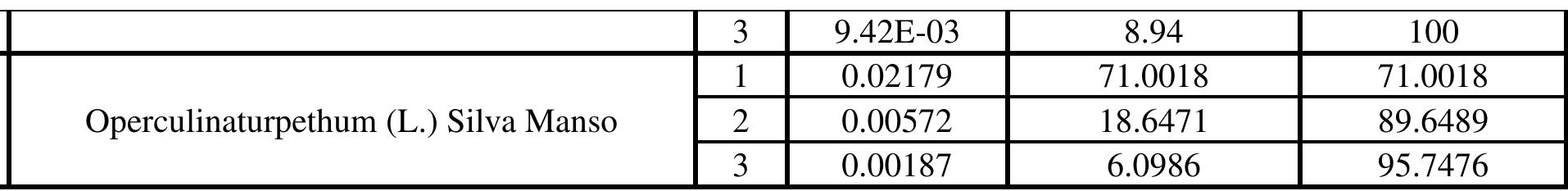
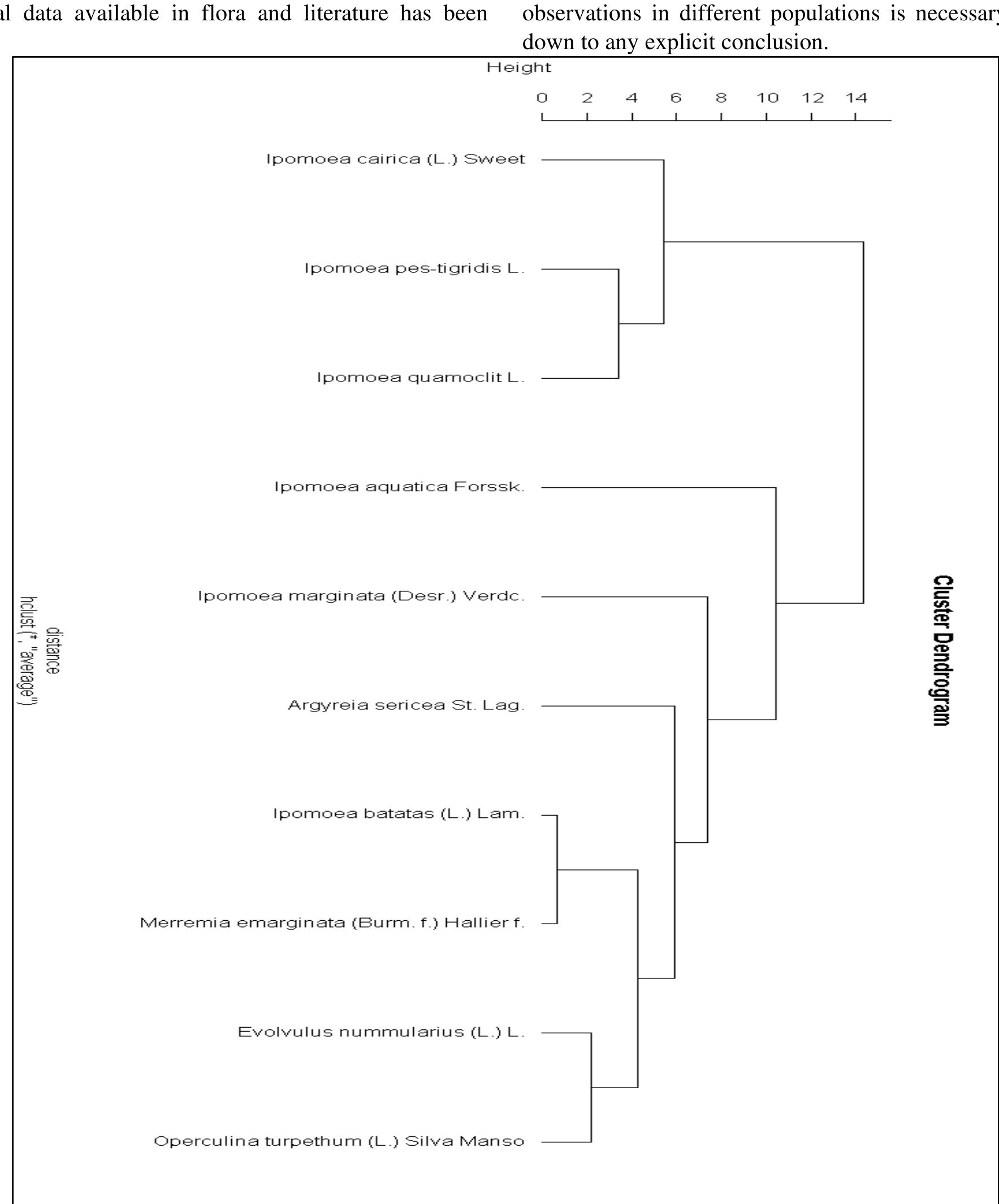
Table 2: Kruskal-Wallis test results morphological level. The shape of mature leaves results from three overlapping, yet distinct processes: first, initiation on the flank of the shoot apical meristem; second, a brief period of primary morphogenesis when the major regions and elements of Shape are defined; and third, a longer period of expansion when initial leaf shape may be altered by allometric expansion (Dengler and Tsukaya, 2001).Cluster dendrogram was constructed after getting the PCA data. The dendrogram (Fig. - 3) depicted that this method can successfully be used in species identification. The cluster dendrogram was constructed by calculating Euclidian distance.
with Merremia has digitate leaves having acuminate apex and Argyria having the deltoid, ovate to lanceolate, apex acute-acuminate, base truncate. Operculina, Evolvulus and I. marginata are in one clade due to ovate, oblong, or oblong elliptic shape and truncate or cordate at the base. The first PC, in A. sericea, variation accounts for 80%, - batatas for 2h, I. - aquatica for 62%, I. ‘Marginata | for 5 8%,
Figure 3: Cluster Dendrogram of PC analysis depicting segregation of species based on leaf shape

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (43)
- Adams D. C., James Rohlf F. and Slice D. E. (2004). Geometric morphometrics: Ten years of progress following the "revolution" Italian Journal of Zoology.
- Adams D.C., Cardini A., Monteiro L.R., O'Higgins P., Rohlf F.J., (2011). Morphometrics and Phylogenetics: principal components of shape from cranial modules are neither appropriate nor effective cladistic characters. Journal of Human Evolution, 60: 240-243.
- Andrade I.M., Mayo S.J., Kirkup D. and Van Den Berg C. (2008). Comparative morphology of populations of Monstera Adans. (Araceae) from natural forest fragments in Northeast Brazil using elliptic Fourier Analysis of leaf outline shape in forest fragment populations of Anthurium sinuatum and A. pentaphyllum (Araceae) from Northeast Brazil. Kew bulletin 65: 3-20
- Austin D.F. (1982) Convolvulaceae. Flora of Venezuela 8 (3):16
- Austin D.F. (1998) Parallel and convergent evolution in the Convolvulaceae. In Biodiversity and taxonomy of tropical flowering plants, 201-234. Mentor Books, Culicut, India
- Bookstein F.L. (1991) Morphometric Tools for Landmark Data. Cambridge Univ. Press, Cambridge.
- Cardini A., Nagorsen D., O'Higgins P., Polly P.D., Thorington J.R.W. (2009) Detecting biological uniqueness using geometric morphometrics: an example case from the Vancouver Island marmot. EcolEtholEvol 21: 209-223.
- Cardini A., Elton S. (2009). The radiation of red colobus monkeys (Primates, Colobinae): morphological evolution in a clade of endangered African primates. Zoological Journal of the Linnean Society, 157: 197-224.
- Cheng X., Ruyter-Spira C. and Bouwmeester H. (2013). The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 4:199.
- Claude J. (2008) Morphometrics with R. Springer Science 1-3pp
- Cooke T. (1958) The Flora of Bombay presidency. Botanical Survey of India (2nd reprint) Kolkata, India.
- Cope, J. S., Corney, D., Clark, J. Y., Remagnino, P. and Wilkin, P. (2012). Plant species identification using digital morphometrics: A review. Expert Syst Appl 39(8): 7562-7573.
- Cronquist A. (1981) An integrated system of classification of flowering plants. New York: Columbia University Press.
- Cronquist A. (1988) The evolution and classification of flowering plants. The New York Botanical Garden, Bronx, New York, USA.
- Demandante, J., Demandante, L., Amamio, V., &Requieron, E. A. (2014). Application of Elliptic Fourier analysis in Sex Identification of Carica papaya Linnaeus (1753) based on Leaf Shape Morphology. 2: 6-12.
- Dengler, N. G., &Tsukaya, H. (2001). Leaf morphogenesis in dicotyledons: Current issues. International Journal of Plant Sciences, 162(3), 459-464.
- Gielis J. (2003) A generic geometric transformation that unifies a wide range of natural and abstract shapes. Ame. J. of Bot. 90: 333-338.
- Hickey L.J. (1973) Classification of the Architecture of Dicotyledonous Leaves. American Journal of Botany 60 (1): 17-33
- Holderegger R., Kamm U., Gugerli F. (2005) Adaptive vs. neutral genetic diversity: implications for landscape genetics Landscape Ecology 21:797-807
- Hooker J.D. (1882) Flora of British India L. Reeve & co. London.
- Ichihashi, Y., Aguilar-Martínez, J. A., Farhi, M., Chitwood, D. H., Kumar, R., Millon, L. V., et al. (2014). Evolutionary developmental transcriptomics reveals a gene network module regulating interspecific diversity in plant leaf shape. Proc. Natl. Acad. Sci. U.S.A. 111, E2616- E2621.
- Iwata H. and Y. Ukai (2002) SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. Jr. of Heredity 93: 384-385.
- Jensen R.J., Ciofani K.M. and Miramontes L.C. (2002) Lines, outlines, and landmarks: morphometric analyses of leaves of Acer rubrum, Acer saccharinum (Aceraceae) and their hybrid. Taxon 51: 475-492.
- Jensen R.J. (2003). The Conundrum of Morphometrics Taxon 52 (4):663-671.
- Klingenberg C. P. (2010). Evolution and Development of shape: Integrating quantitative approaches. Nat. Rev. Genet 11: 623-635
- Kuhl F.P. and Giardina C.R. (1982) Elliptic Fourier features of a closed contour. Comput Graph Imag Process 18:236-258.
- Lestrel P.E. (1997). Introduction. Pp. 3-21 in: Lestrel PE (ed.), Fourier Descriptors and their Applications in Biology. Cambridge Univ. Press, Cambridge.
- Little, S. A., Kembel, S. W., and Wilf, P. (2010). Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history. PLoS ONE 5:e15161.
- Mabberly D.J. (2008). The plant book: A portable dictionary of plants, their distribution and uses. (3rd ed.) Cambridge University press Cambridge.
- MacLeod, N. 2002. Phylogenetic signals in morphometric data. Pp. 100-138 in: MacLeod, N. &Forey, P. L. (eds.), Morphology, Shape and Phylogeny. Taylor & Francis, London
- MacLeod N., Benfield M. and Culverhouse P. (2010) Time to automate identification. Nature 467: 154-155.
- MacLeod N. 2005. Shape models as a basis for morphological analysis in paleobiological systematics: dicotyledonous leaf physiography. Bull. of Ame. Paleo. 369: 219-238.
- Marcus L.F., Corti M. (1996) Overview of the new, or geo-metric morphometrics. Pp. 1-13 in: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE (eds.) Advances in Morphometrics. Plenum Press, New York.
- Marcus, L.F. (1990). Traditional morphometrics. In Proceedings of the Michigan Morphometrics Workshop.
- Munné-Bosch S, Maren Müller (2013) Hormonal cross-talk in plant development and stress responses. Front. Pl. Sci. 4:529.
- Neff, N. A. and L. F. Marcus. (1980). A Survey of Multivariate Methods for Systematics. New York: privately published.
- Rohlf F. (1998). On Applications of Geometric Morphometrics to Studies of Ontogeny and Phylogeny. Systematic Biology. (47).1:147- 158.
- Royer, D. L., Meyerson, L. A., Robertson, K. M., and Adams, J. M. (2009). Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS ONE 4:e7653.
- Shah G. L. (1978). Flora of Gujarat State. Vallabh Vidyanagar [India: Sardar Patel University.
- Slice D. E. (1996). Introduction to Landmark Methods. In Advances in Morphometrics.
- Viscosi V., Fortini P. (2011) Leaf shape variation and differentiation in three sympatric white oak species revealed by elliptic Fourier analysis. Nordic J Bot 29:632-640.
- Viscosi V, Cardini A (2012) Correction: Leaf Morphology, Taxonomy and Geometric Morphometrics: A Simplified Protocol for Beginners. PLoS ONE 7(3): e25630.
- William H. Kruskal & W. Allen Wallis (1952) Use of Ranks in One- Criterion Variance Analysis, Journal of the American Statistical Association, 47:260, 583-621.