Design and Analysis of Phase Change Material based thermal energy storage for active building cooling: a Review (original) (raw)
Abstract
Phase Change Materials (PCMs) are "latent" thermal storage materials. They use chemical bonds to store and release heat. The thermal energy transfer occurs when a material changes from a solid to a liquid or from a liquid to a solid form. This is called a change in state or "phase." Initially, these solid-liquid PCMs perform like conventional storage materials; their temperature rises as they absorb solar heat. Unlike conventional heat storage materials, when PCMs reach the temperature at which they change phase (their melting point), they absorb large amounts of heat without getting hotter. When the ambient temperature in the space around the PCM material drops, the Phase Change Material solidifies, releasing its stored latent heat. PCMs absorb and emit heat while maintaining a nearly constant temperature. Within the human comfort and electronic-equipment tolerance range of 20°C to 35°C, latent thermal storage materials are very effective. They can be used for equalization of day & night temperature and for transport of refrigerated products. In the proposed project heat of fusion of Cacl2. 6H2o as PCM is used for cooling water during night and this cooled water is used as circulating medium trough fan coil unit, air trough FCU will get cooled by transferring heat to water and fresh & cool air will be thrown in a room. In the proposed project FREE COOLING & ACTIVE BUILDING COOLING concepts of Thermal Energy Storage are used in combine
FAQs
AI
What energy savings does the PCM-based system achieve compared to conventional cooling systems?add
The system reduces energy consumption and costs by 40%, providing significant economic benefits. This is achieved by eliminating the need for conventional air conditioning compressors.
What specific properties make CaCl2.6H2O suitable as a phase change material?add
CaCl2.6H2O exhibits a melting temperature of 28-30°C and high heat of fusion. Additionally, it possesses large thermal conductivity and stability over multiple freeze/melt cycles.
How does the phase change process of CaCl2.6H2O function in thermal storage?add
During cooling, CaCl2.6H2O absorbs latent heat as it transitions to solid, maintaining low temperatures. This process facilitates efficient energy storage and retrieval during discharging.
What impacts does this PCM system have on indoor air quality?add
The system improves indoor air quality by effectively controlling humidity levels. This is crucial for achieving comfortable and healthy living conditions in buildings.
How does the use of this PCM system contribute to environmental sustainability?add
Utilizing this PCM system minimizes GHG and CFC emissions associated with traditional cooling methods. It also reduces reliance on fossil fuels, further promoting eco-friendliness.
Figures (6)
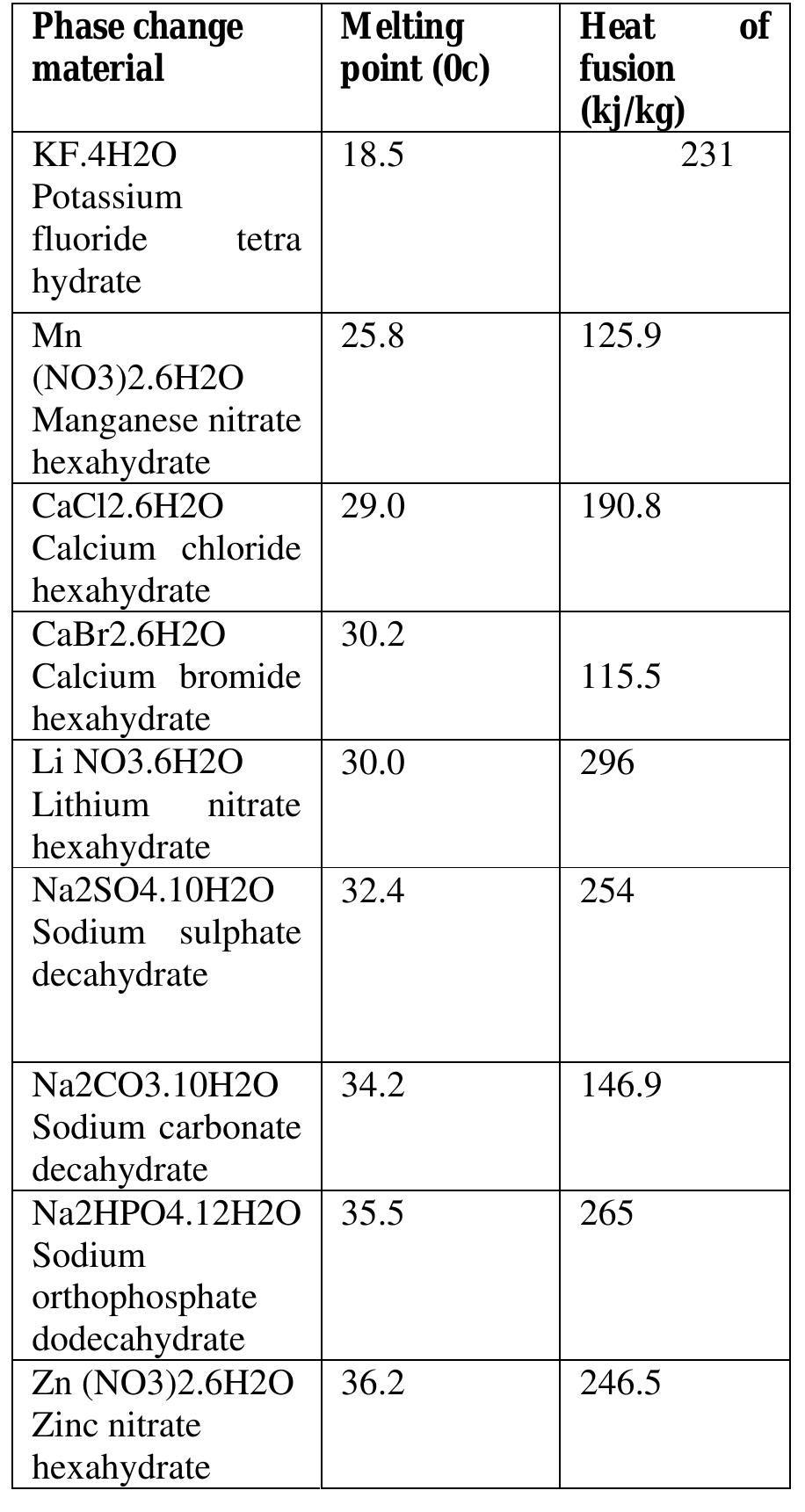
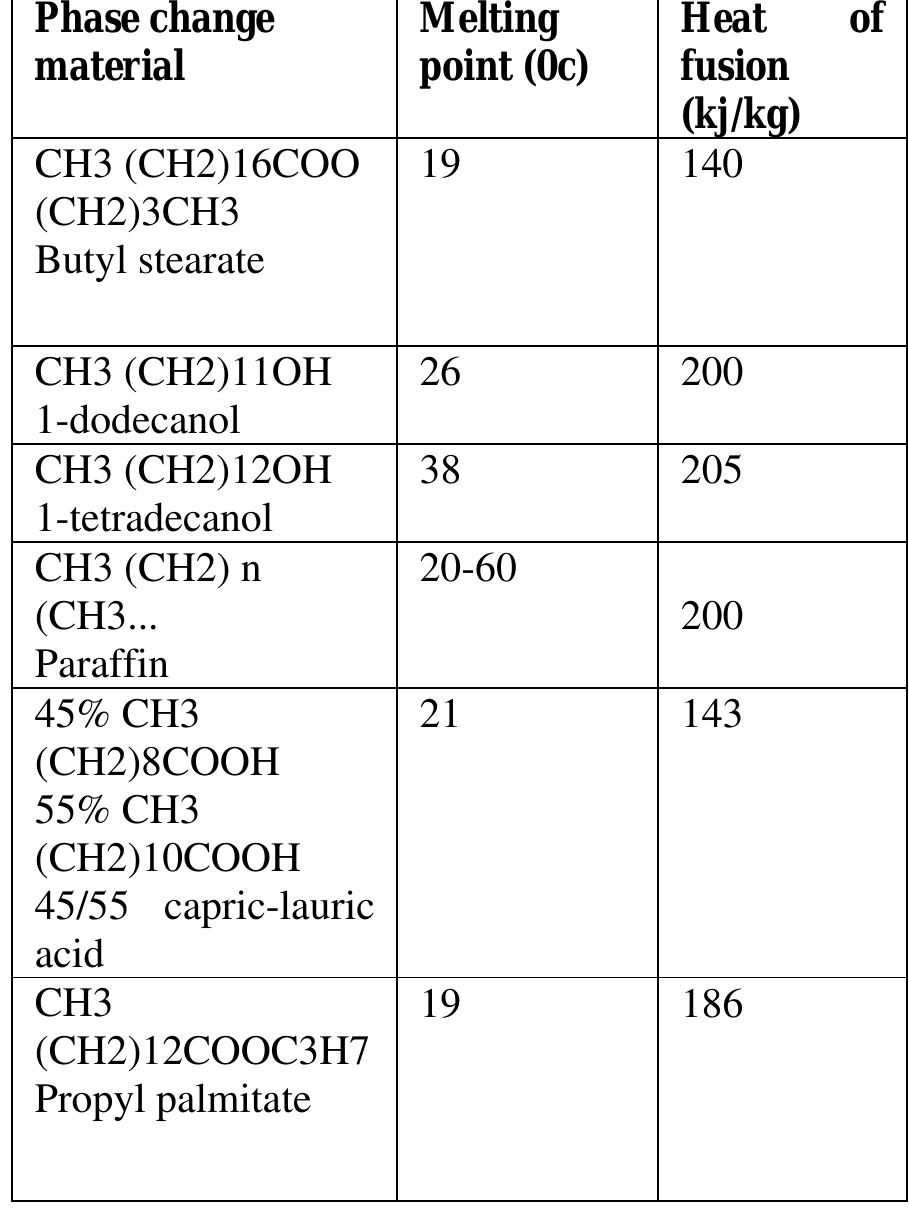
(ii) Organic PCMs Table 1: Salt hydrate PCMs (typical values) baal Organic PCMs have a number of characteristics which render them useful for latent heat storage. They are more chemically stable than inorganic substances, they melt congruently and super cooling does not pose as a significant problem. Although the initial cost of organic PCMs is higher than that of the inorganic type, the installed cost is competitive. However, these organic materials do have their quota of unsuitable properties. Of the most significant of these characteristics, they are flammable and they may generate harmful fumes on combustion. Other problems, which can arise in a minority of cases, are a reaction with the products of hydration in concrete, thermal oxidative ageing, odour and an appreciable volume change. The most promising selection of these organic PCMs is shown in Table 2.
Selection of PCM Table 2: Salt hydrate PCMs (typical values)
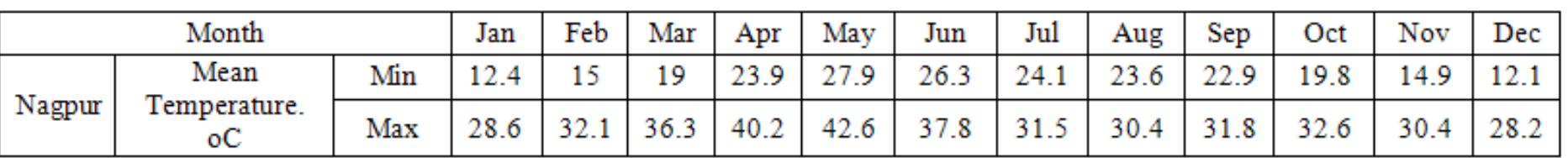
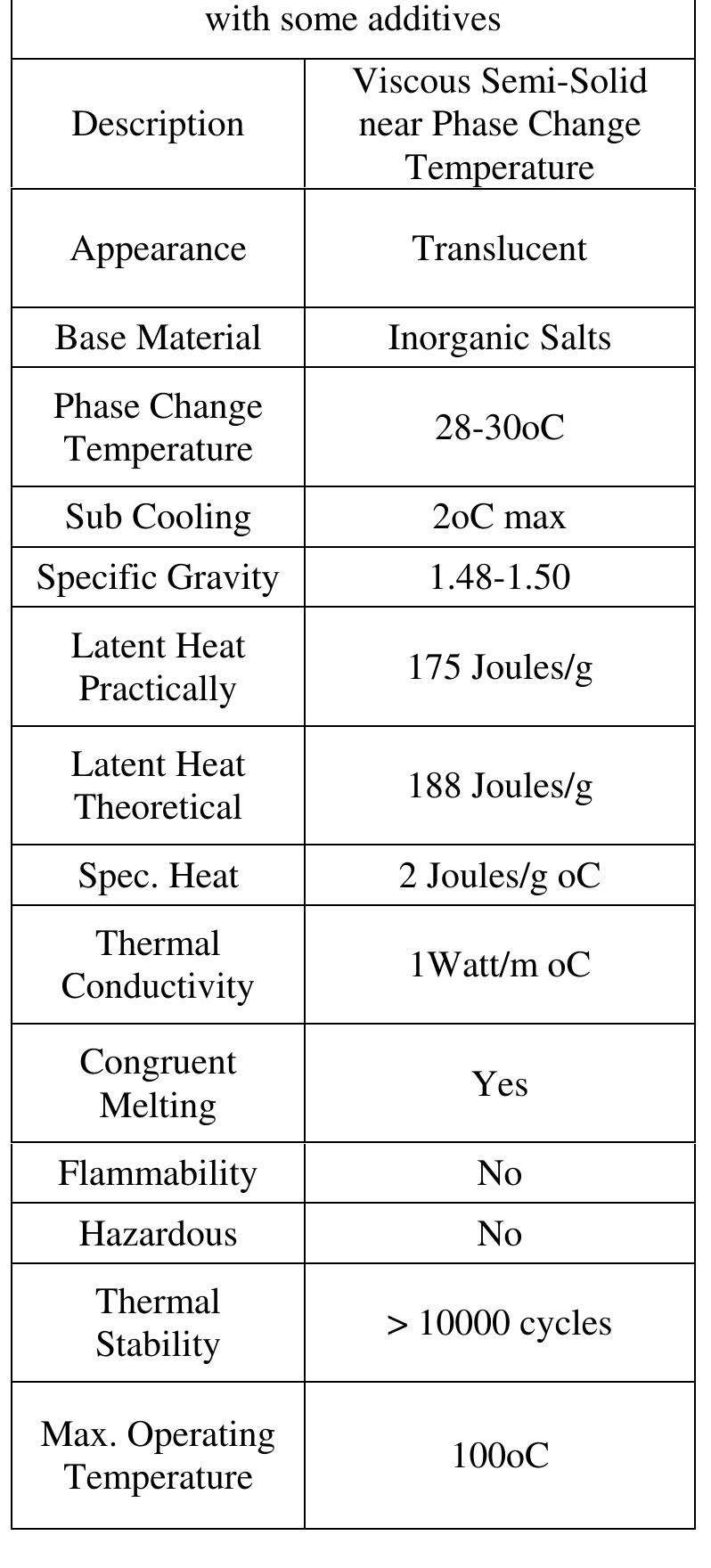
Cacl2.6h20 (calcium chloride hexa- hydrate) is most suitable for climatic conditions of Nagpur city; it is easily available, economical, non-toxic, non-dangerous, non-corrosive, and chemically stable. It have limited changes in density to avoid problems with the storage tank, low vapour pressure, favorable phase equilibrium. Table 3: An average temp range 2010 Thermo-chemical properties of Cacl2.6h2o0 (calcium chloride hexa- hydrate) are given in table 4
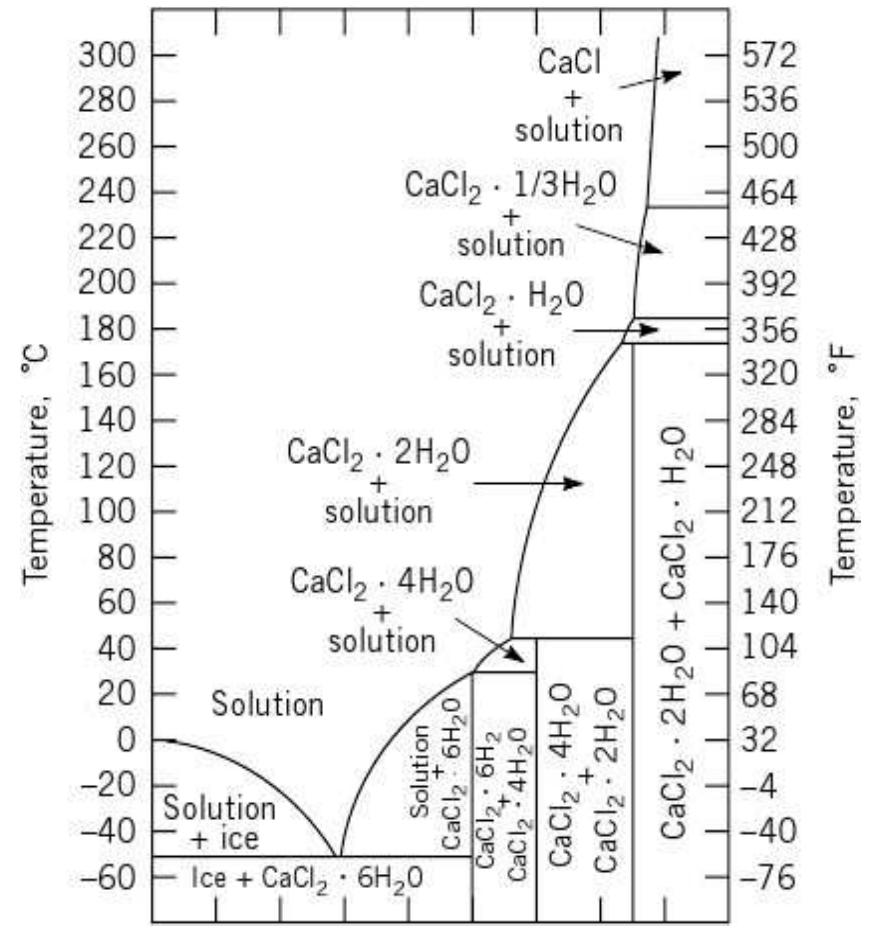
aniius J & £10400 a: CbSEke BEE WY CAEULUSEE WY £202 AU WY CEU My vuuuiis pure water freezes to ice at 0°C. If CaCl2 or another solute is added to water, the freezing point of the solution will be lower than 0°C. As the graph below shows, ice will form at -20°C in a 20 mass % solution of CaCl2. This phenomenon is called a freezing point depression. It can be explained from changes in chemical potentials Table 4: thermo-physical properties of cacl2.6h20
Fig-Phase diagram for the binary CaCl2 - H20 system Nitin .D. Patil et al. / International Journal of Engineering Science and Technology (IJEST)
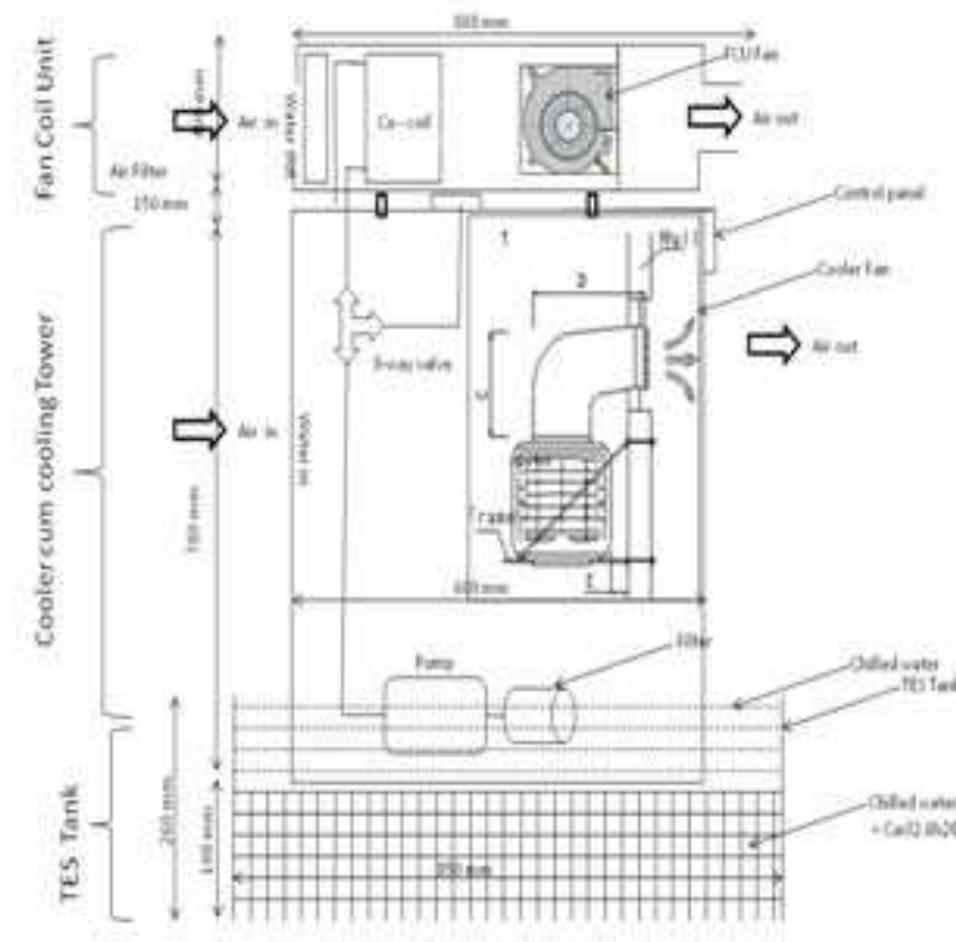
Experimental set up: Fig — Cacl2.6h20 based TES System for active building cooling purpose

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (17)
- Thermal energy storage design manual by www.pcmproducts.net.
- A review of materials, heat transfer and phase change problem formulation 1 for latent heat thermal energy storage systems (LHTESS)
- Francis Agyenim , Neil Hewitt , Philip Eames , Mervyn Smyth, Renewable 1 and Sustainable Energy Reviews 14 (2010) 615- 628
- Thermal cycle testing of calcium chloride hexahydrate as a possible PCM for 1 latent heat storage
- V.V. Tyagi _, D. Buddhi, Solar Energy Materials & Solar Cells 92 (2008) 1
- Review on thermal energy storage with phase change: materials, 1 heat transfer analysis and applications
- Bel_en Zalba , Jos_e Ma Mar_ın a, Luisa F. Cabeza b,Harald 1 1 111Mehling , Applied Thermal Engineering 23 (2003) 251-283
- PCM thermal storage in buildings: A state of art
- Vineet Veer Tyagi, D. Buddhi, Renewable and Sustainable Energy Reviews 11 (2007) 1146-1166
- Trane Cooling system manual.
- R. Velraj, A. Pasupathy, "Phase change material based thermal storage for energy conservation in building Architecture", Institute for energy studies CEG, Anna University, Chennai
- M. faith Demirbas, researcher of energy technology Trabzon, Turkey. Energy sources, part B, 1:85-95, 2006.
- Ruth Kelly B.Sc. (Eng), AMEC Design. "Latent heat storage in building materials".
- J.P.Holman, "A text book of heat and mass transfer." Tata MC Graw Hill publication.
- C. Arkar, S. Medved, Free cooling of a building using PCM heat storage integrated into the ventilation system. Solar Energy 81 (2007) 1078-1087
- PCM for cooling system".
- V.V.Tyagi, D.Buddhi, "Thermal cycle testing of calcium chloride hexahydrate as a possible PCM for latent heat storage". Elsevier publication, February 2008.