Green synthesis of nanoparticles and its potential application (original) (raw)
Green synthesis of nanoparticles and its potential application
Imtiyaz Hussain ⋅\cdot N. B. Singh ⋅\cdot
Ajey Singh ⋅\cdot Himani Singh ⋅\cdot S. C. Singh
Received: 7 August 2015 / Accepted: 23 December 2015
© Springer Science+Business Media Dordrecht 2015
Abstract
Nanotechnology is a new and emerging technology with wealth of applications. It involves the synthesis and application of materials having one of the dimensions in the range of 1−100 nm1-100 \mathrm{~nm}. A wide variety of physico-chemical approaches are being used these days for the synthesis of nanoparticles (NPs). However, biogenic reduction of metal precursors to produce corresponding NPs is eco-friendly, less expensive, free of chemical contaminants for medical and biological applications where purity of NPs is of major concern. Biogenic reduction is a “Bottom Up” approach similar to chemical reduction where a reducing agent is replaced by extract of a natural products with inherent stabilizing, growth terminating and capping properties. Furthermore, the nature of biological entities in different concentrations in combination with reducing organic agents influence the size and shape of NPs. Present review focuses on microbes or plants based green synthesis of Ag,Au\mathrm{Ag}, \mathrm{Au}, Cu,Fe,Pd,Ru,PbS,CdS,CuO,CeO2,Fe3O4,TiO2\mathrm{Cu}, \mathrm{Fe}, \mathrm{Pd}, \mathrm{Ru}, \mathrm{PbS}, \mathrm{CdS}, \mathrm{CuO}, \mathrm{CeO}_{2}, \mathrm{Fe}_{3} \mathrm{O}_{4}, \mathrm{TiO}_{2}, and ZnO NPs and their potential applications.
[1]Keywords Biological applications ⋅\cdot Biogenic reduction ⋅\cdot Ecofriendly ⋅\cdot Metal nanoparticles ⋅\cdot Nanotechnology ⋅\cdot Plants ⋅\cdot Purity
Introduction
Nanoparticles (NPs) having one of the dimension in the range of 1−100 nm1-100 \mathrm{~nm} act as a bridge between bulk materials and atomic or molecular structures (Kaushik et al. 2010). They possess remarkable and interesting properties owing their small sizes, large surface area with free dangling bonds and higher reactivity over their bulk cousins (Kubik and Sugisaka 2002; Daniel and Astruc 2004; Zharov et al. 2005). Since the nineteenth century scientists have been well aware of the ability of biological entities to reduce metal precursors but the mechanisms are still unexplored. The progress of efficient green synthesis utilizing natural reducing, capping and stabilizing agents without the use of toxic, expensive chemicals and high energy consumption have attracted researchers towards biological methods (Mukherjee et al. 2001; Mohanpuria et al. 2008; Korbekandi et al. 2009; Luangpipat et al. 2011; Dhillon et al. 2012; Arumugama et al. 2015).
Rapid industrialization, urbanization and population explosion are resulting in deterioration of earth atmosphere and a huge amount of hazardous and unwanted substances are being released. It is now high time to learn about the secrets that are present in the
- I. Hussain ⋅\cdot N. B. Singh ( ⊠\boxtimes ) ⋅\cdot A. Singh ⋅\cdot H. Singh Plant Physiology Laboratory, Department of Botany, University of Allahabad, Allahabad, UP 211002, India e-mail: singhnb166@gmail.com
S. C. Singh
Laser Spectroscopy Laboratory, Department of Physics, University of Allahabad, Allahabad, UP 11002, India ↩︎
nature and its natural products which lead to advancements in the synthesis processes of NPs. Furthermore, NPs are widely applied to human contact areas and there is a growing need to develop processes for synthesis that do not use harsh toxic chemicals. Therefore, green/biological synthesis of NPs is a possible alternative to chemical and physical methods.
The first question related with production of green nanomaterials is “Why are biological-synthesized NPs so interesting and gaining importance nowadays?” The unique properties of the NPs synthesized by biological methods are preferred over nanomaterials produced from physico-chemical methods. NPs may be synthesized following physico-chemical methods (Singh et al. 2015a). However, these methods are capital extensive with many problems including use of toxic solvents, generation of hazardous byproducts and the imperfection of the surface structure ( Li et al. 2011). Chemical methods are generally composed by more than one chemical species or molecules that could increase the particle reactivity and toxicity and might harm human health and the environment due to the composition ambiguity and lack of predictability (Li et al. 2011).
The particles produced by green synthesis differ from those using physico-chemical approaches. Green synthesis, a bottom up approach, is similar to chemical reduction where an expensive chemical reducing agent is replaced by extract of a natural product such as leaves of trees/crops or fruits for the synthesis of metal or metal oxide NPs. Biological entities possess a huge potential for the production of NPs. Biogenic reduction of metal precursors to corresponding NPs is eco-friendly (Jayaseelana et al. 2012), sustainable (Gopinath et al. 2014), free of chemical contamination (Chandran et al. 2006; Huang et al. 2007), less expensive (Mittal et al. 2013) and can be used for mass production (Iravani 2011). Moreover, the biological production of NPs allows recycle of expensive metal salts like gold and silver contained in waste streams. These metals have limited resources and have fluctuating prices (Wang et al. 2009). We may get green NPs with the desired properties. The biological molecules, mostly proteins, enzymes, sugars and even whole cells, that stabilize NPs easily allow NPs to interact with other biomolecules and thus increase the antimicrobial activity by improving the interactions with microorganisms (Botes and Cloete 2010). The biological formation of NPs permits easy
separation of the NPs from the reaction media or upconcentration by centrifugation (Sintubin et al. 2009). Biogenic silver NPs when compared to chemicallyproduced NPs showed 20 times higher antimicrobial activity (Sintubin et al. 2011). The choice of plant extracts to produce NPs is based on the added value of the biological material itself. The algal cells of Spirulina platensis was chosen because in addition to possessing reducing agent it also exhibits pharmaceutical and nutraceutical properties (Govindaraju et al. 2008).
Unicellular bacteria and extracts of multi-cellular eukaryotes in the reaction processes reduce metal precursors into NPs of desire shapes and sizes (Kaushik et al. 2010). In addition to this, biological entities possess capping and stabilizing agents required in as growth terminator and for inhibiting aggregation/agglomeration process (Kharissova et al. 2013). The nature of biological entities and its concentrations in combination with organic reducing agents influence the size and shape of NPs (Aromal et al. 2012). Moreover, size and shape of NPs strongly depend on the growth medium parameters such as pH , temperature, salt concentration and exposure time (Gericke and Pinches 2006; Dwivedi and Gopal 2010). Bio-reduction of metal precursors takes place either in vitro or in vivo for the synthesis of nanomaterials. However, enzymes, proteins, sugars, and phytochemicals, like flavonoids, phenolics, terpenoids, cofactors etc., mainly act as reducing and stabilizing agents (Kaushik et al. 2010; Kharissova et al. 2013).
The in vivo production of NPs have been reported using bacteria, yeast, fungi, algae and plants (Torresdy et al. 2003; Narayanan and Sakthivel 2010; Duran et al. 2011; Lloyd et al. 2011; Kharissova et al. 2013). Mostly biological extracts are used for in vitro synthesis, which involves the purification of bio-reducing agents and mixing it into an aqueous solution of the relevant metal precursor in controlled manner. The reaction occurs spontaneously at room temperature (Rajakumara et al. 2012) but sometimes additional heating and stirring are needed (Sankar et al. 2014). Among the biological entities mentioned above, plants or their extracts seem to be the best agents because they are easily available, suitable for mass production of NPs and their waste products are eco-friendly (Lee et al. 2011) unlike some microbial extracts.
Despite a great deal of research in nanotechnology using physico-chemical approaches, synthesis of
silver (Ag)(\mathrm{Ag}) and gold (Au)(\mathrm{Au}) NPs are widely exploited using green synthesis. However, a relatively modest number of studies have attempted to elucidate the biosynthesis and potential applications of other metallic and semiconductor NPs. This review presents an overview on biosynthesis of various metallic and semiconductor NPs viz. Cu,Fe,Pd,Ru,PbS,CdS\mathrm{Cu}, \mathrm{Fe}, \mathrm{Pd}, \mathrm{Ru}, \mathrm{PbS}, \mathrm{CdS}, CuO,CeO2,Fe3O4,TiO2\mathrm{CuO}, \mathrm{CeO}_{2}, \mathrm{Fe}_{3} \mathrm{O}_{4}, \mathrm{TiO}_{2}, and ZnO NPs with an emphasis on their applications in biotechnology.
Green production of nanoparticles and their impacts
NPs synthesis may generally follow either a “Top Down” approach or “Bottom Up” approach (Fig. 1). In “Top Down” approach NPs are produced by size
reduction and is achieved by various physical and chemical methods (Singh et al. 2010). In “Bottom Up” synthesis, NPs are produced from small entities, like atoms and molecules, where the main reaction is reduction/oxidation. In this greener route, NPs are obtained with minimum defects and homogenous chemical composition. In the synthesis of NPs by biological methods, microorganisms as well as plant extracts are widely used (Dameron et al. 1989; Sweeney et al. 2004; Bharde et al. 2005; Vigneshwaran et al. 2006; Lee et al. 2011; Joglekar et al. 2011; Gopinath et al. 2014). The possible mechanism of synthesis of NPs by biological method is presented in Fig. 2.
The specific characteristics of the organisms such as biochemical pathways, phytochemical contents and enzyme activities and conditions for cell growth as
Fig. 1 Generalized flow chart of various physicochemical approaches of nanoparticles synthesis with highlighting of biological synthesis
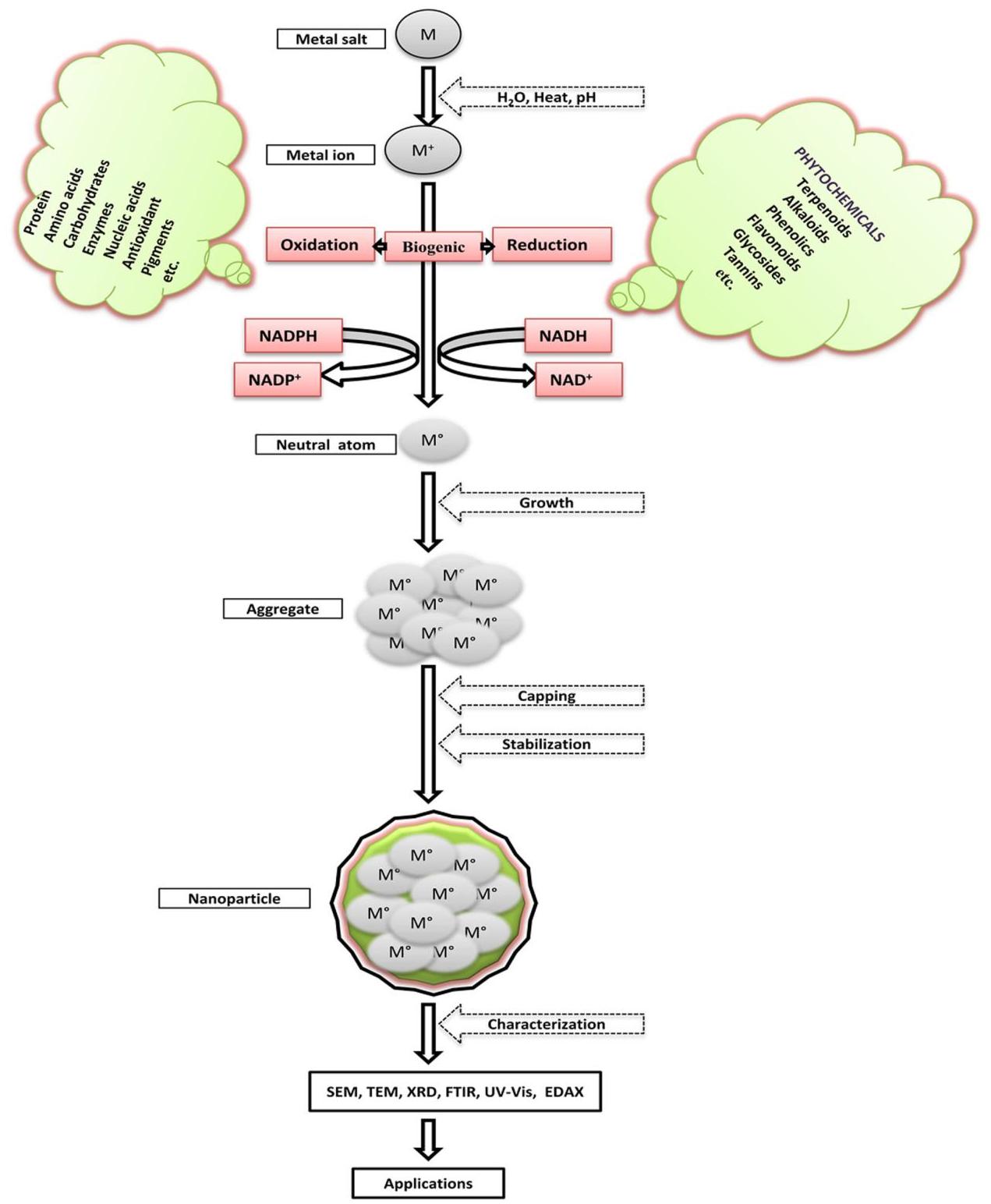
Fig. 2 Diagram summarizing the possible mechanism of biologically mediated synthesis of nanoparticles. MM metal salt, M+M^{+}M+Metal ion, M∗M^{*} neutral atom
well as optimal reaction are to be considered for selection of the best organisms or its extracts (Ahmad et al. 2003). Some organisms used for the production of NPs are listed in the Tables 1 and 2.
Titanium dioxide (TiO2)\left(\mathrm{TiO}_{2}\right)
Synthesis of TiO2NPs\mathrm{TiO}_{2} \mathrm{NPs} (36-38 nm, spherical) using a leaf extract of Eclipta prostrata was carried out at
Table 1 Biological entities which synthesize metal oxides nanoparticles with their size, shape and brief experiments
| Biological entities | Precursors, conditions | NPs a^{\mathrm{a}}, size and shape | Key aspects | Refb\operatorname{Ref}^{\mathrm{b}} |
|---|---|---|---|---|
| Aeromonas hydrophila (bacterium) | ZnO,24 h\mathrm{ZnO}, 24 \mathrm{~h} at 30∘C30^{\circ} \mathrm{C} | ZnO,57−72 nm, spherical \begin{aligned} & \mathrm{ZnO}, \\ & 57-72 \mathrm{~nm} \text {, } \\ & \text { spherical } \end{aligned} | Exhibited antimicrobial activity against both bacteria (Pseudomonas aeruginosa) and fungi (Aspergillus flavus) | Jayaseelana et al. (2012) |
| Aloe barbadensis (plant) Source gel | Zn(NO3)2,5−6 h at 150∘C\begin{aligned} & \mathrm{Zn}\left(\mathrm{NO}_{3}\right)_{2}, 5-6 \mathrm{~h} \text { at } \\ & 150^{\circ} \mathrm{C} \end{aligned} | ZnO,25−40 nm\begin{aligned} & \mathrm{ZnO}, \\ & 25-40 \mathrm{~nm} \end{aligned} | Size control by varying concentrations of leaf broth solution | Gunalan et al. (2011) |
| Aspergillus flavus (fungus) | TiO2\mathrm{TiO}_{2} at 37∘C37^{\circ} \mathrm{C} | TiO262−74 nm\begin{aligned} & \mathrm{TiO}_{2} \\ & 62-74 \mathrm{~nm} \end{aligned} oval | Effective against S. aureus | Rajakumara et al. (2012) |
| Bacillus mycoides (bacterium) | TiO(OH)2,12 h at 37∘C\begin{aligned} & \mathrm{TiO}(\mathrm{OH})_{2}, 12 \mathrm{~h} \text { at } \\ & 37^{\circ} \mathrm{C} \end{aligned} | TiO240−60 nm\begin{aligned} & \mathrm{TiO}_{2} \\ & 40-60 \mathrm{~nm} \end{aligned} spherical | Used to synthesize green solar cell and effective against E. coli (BW25113) | Aenishanslins et al. (2014) |
| Bacillus subtilis (bacterium) | K2 F6Ti,48 h\begin{aligned} & \mathrm{K}_{2} \mathrm{~F}_{6} \mathrm{Ti}, 48 \mathrm{~h} \end{aligned} | TiO210−30 nm\begin{aligned} & \mathrm{TiO}_{2} \\ & 10-30 \mathrm{~nm} \end{aligned} spherical | Suppress aquatic biofilm growth | Dhandapani et al. (2012) |
| Bacteria strains NS2 and NS6 (bacterium) | PbCl2,CaSO4,24 h at 37∘C\begin{aligned} & \mathrm{PbCl}_{2}, \mathrm{CaSO}_{4}, 24 \mathrm{~h} \text { at } \\ & 37^{\circ} \mathrm{C} \end{aligned} | PbS,40−70 nm\mathrm{PbS}, 40-70 \mathrm{~nm} | Bioremediation without producing toxic chemicals to the environment | Singh and Naraa (2013) |
| Cassia alata (plant) Source flower | CuSO4,45 min at 80∘C\begin{aligned} & \mathrm{CuSO}_{4}, 45 \mathrm{~min} \text { at } \\ & 80^{\circ} \mathrm{C} \end{aligned} | CuO,110−280 nm\begin{aligned} & \mathrm{CuO}, \\ & 110-280 \mathrm{~nm} \end{aligned} spherical | Pale green colour after 2 h indicated formation of NPs and may have wide application in medicine | Jayalakshmi and Yogamoorthi (2014) |
| Cassia auriculata (plant) Source flower | ZnNO3,60−80∘C\mathrm{ZnNO}_{3}, 60-80^{\circ} \mathrm{C} | ZnO | Renewable leaf extract of test plant can be used as an effective stabilizing as well as reducing agent for the synthesis of NPs | Ramesh et al. (2014) |
| Catharanthus roseus (Vinca rosea) (plant) Source leaves | TiO2,4 h at 50∘C\begin{aligned} & \mathrm{TiO}_{2}, 4 \mathrm{~h} \text { at } 50^{\circ} \mathrm{C} \end{aligned} | TiO225−110 nm\begin{aligned} & \mathrm{TiO}_{2} \\ & 25-110 \mathrm{~nm} \end{aligned} irregular | Effective against Hippobosca maculate and Bovicola ovis | Velayutham et al. (2012) |
| Euphorbia condylocarpa (plant) Source root | PdCl2 and FeCl36H2O, at 60∘C\begin{gathered} \mathrm{PdCl}_{2} \text { and } \\ \mathrm{FeCl}_{3} 6 \mathrm{H}_{2} \mathrm{O} \text {, at } \\ 60^{\circ} \mathrm{C} \end{gathered} | Pd/Fe3O4,Avg39 nm\begin{gathered} \mathrm{Pd} / \mathrm{Fe}_{3} \mathrm{O}_{4}, \mathrm{Avg} \\ 39 \mathrm{~nm} \end{gathered} | Magnetically recoverable and recyclable catalyst | Nasrollahzdeha et al. (2015a) |
| Gloriosa superba (plant) source leaves | CuNO3,3−4 min at 400∘C\begin{aligned} & \mathrm{CuNO}_{3}, 3-4 \mathrm{~min} \text { at } \\ & 400^{\circ} \mathrm{C} \end{aligned} | CuO,5−10 nm, spherical \begin{aligned} & \mathrm{CuO}, 5-10 \mathrm{~nm} \text {, } \\ & \text { spherical } \end{aligned} | Effective against S. aureus and Klebsiella aeroegenes | Naikaa et al. (2015) |
| Gloriosa superba (plant) Source leaves | CeCl3,4−6 h\mathrm{CeCl}_{3}, 4-6 \mathrm{~h} at 80∘C80^{\circ} \mathrm{C} | CeO2,Avg5 nm\begin{aligned} & \mathrm{CeO}_{2}, \mathrm{Avg} \\ & 5 \mathrm{~nm} \end{aligned} spherical | Uneven ridges and oxygen defects made NPs toxicological behaviour | Arumugama et al. (2015) |
| Camellia sinensis (plant) Source leaves | Zn(O2CCH3)2(H2O)2 Over night at 60∘C\begin{aligned} & \mathrm{Zn}\left(\mathrm{O}_{2} \mathrm{CCH}_{3}\right)_{2}\left(\mathrm{H}_{2} \mathrm{O}\right)_{2} \\ & \text { Over night at } 60^{\circ} \mathrm{C} \end{aligned} | ZnO,Avg16 nm\begin{aligned} & \mathrm{ZnO}, \mathrm{Avg} \\ & 16 \mathrm{~nm} \end{aligned} | Effective against Klebsiella pneumonia | Senthilkumar and Sivakumar (2014) |
| Gum karaya (plant) Source Gum | CuCl2⋅2H2O,1 h at 75∘C\begin{aligned} & \mathrm{CuCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}, 1 \mathrm{~h} \text { at } \\ & 75^{\circ} \mathrm{C} \end{aligned} | CuO,Avg4.8 nm\begin{aligned} & \mathrm{CuO}, \mathrm{Avg} \\ & 4.8 \mathrm{~nm} \end{aligned} | Antimicrobial activity against E. coli (MTCC 443) | Vellora et al. (2013) |
| Humicola sps (fungus) | CeN3O9⋅6H2O, at 50∘C\begin{aligned} & \mathrm{CeN}_{3} \mathrm{O}_{9} \cdot 6 \mathrm{H}_{2} \mathrm{O} \text {, at } \\ & 50^{\circ} \mathrm{C} \end{aligned} | CeO212−20 nm\begin{aligned} & \mathrm{CeO}_{2} \\ & 12-20 \mathrm{~nm} \end{aligned} spherical | Capping protein made NPs H2O\mathrm{H}_{2} \mathrm{O} disperse and favourable for medical applications | Khan and Ahmad (2013) |
Table 1 continued
| Biological entities | Precursors, conditions | NPs a^{\mathrm{a}}, size and shape | Key aspects | Ref b^{\mathrm{b}} |
|---|---|---|---|---|
| Malva sylvestris (plant) Source leaves | CuCl2⋅2H2O,2 min at 80∘C\begin{aligned} & \mathrm{CuCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}, 2 \mathrm{~min} \text { at } \\ & 80^{\circ} \mathrm{C} \end{aligned} | CuO, 5-30 nm, spherical | Effective against both gram +ve and -ve bacteria | Awwad et al. (2015) |
| Phyllanthus amarus (plant) Source leaves | CuSO4,7 h\mathrm{CuSO}_{4}, 7 \mathrm{~h} at 130∘C130^{\circ} \mathrm{C} | CuO, 20 nm , spherical | Effective than rifampicin against B. subtilis | Acharyulu et al. (2014) |
| Rhodobacter sphaeroides (bacterium) | CdCl2,36−48 h at 30∘C\begin{aligned} & \mathrm{CdCl}_{2}, 36-48 \mathrm{~h} \text { at } \\ & 30^{\circ} \mathrm{C} \end{aligned} | CdS, 2.3−36.8 nm\begin{aligned} & \text { CdS, } \\ & 2.3-36.8 \mathrm{~nm} \end{aligned} Spherical | Size of NPs increased with the increase in culture time | Bai et al. (2009) |
| Oryza sativa (plant) Source straw | TiO2(OH)2, Until gel formed at 80∘C\begin{aligned} & \mathrm{TiO}_{2}(\mathrm{OH})_{2}, \text { Until gel } \\ & \text { formed at } 80^{\circ} \mathrm{C} \end{aligned} | TiO213±3.3 nm\begin{aligned} & \mathrm{TiO}_{2} \\ & 13 \pm 3.3 \mathrm{~nm} \end{aligned} | Modification of the pore volume and size and decreasing the particle size enhanced the surface area which made TiO2\mathrm{TiO}_{2} NPs highly potential photocatalyst | Ramimoghadam et al. (2014) |
a{ }^{a} Nanoparticles
b{ }^{\mathrm{b}} References
room temperature from titanium hydroxide [TiO(OH)2]\left[\mathrm{TiO}(\mathrm{OH})_{2}\right] solution (Rajakumar et al. 2012). The reduction was attributed to the stretching of carboxyl (COOH)(\mathrm{COOH}) and amine (−NH2)\left(-\mathrm{NH}_{2}\right) groups present in the extract. The authors of this study observed that the produced NPs may have a great scope in the fields of coating, cosmetics, food additive, etc. Sankar et al. (2014) reported the possibility of amide, carboxyl and nitro groups, from Azadirachta indica leaf extract in the synthesis of TiO2NPs\mathrm{TiO}_{2} \mathrm{NPs} of 124 nm average size with spherical shape. They also investigated the role of NPs as effective photo-catalyst towards the remediation of pollution.
Environmental isolate Bacillus mycoides was used to produced TiO2NPs(40−60 nm\mathrm{TiO}_{2} \mathrm{NPs}(40-60 \mathrm{~nm}, spherical) at room temperature in the presence of TiO(OH)2\mathrm{TiO}(\mathrm{OH})_{2} (Aenishanslins et al. 2014). These NPs are now used in construction of green solar cells and were found to inhibit the growth of E. coli. In a separate report of bacterial synthesis of NPs, Bacillus subtilis, when exposed to potassium hexafluorotitanate (K2TiF6)\left(\mathrm{K}_{2} \mathrm{TiF}_{6}\right), reduced the metal precursors to TiO2NPs(10−30 nm\mathrm{TiO}_{2} \mathrm{NPs}(10-30 \mathrm{~nm}, spherical) with improved poly-dispersity (Dhandapani et al. 2012). This study demonstrated that H2O2\mathrm{H}_{2} \mathrm{O}_{2} in the vicinity of NPs suppressed the growth of aquatic biofilm.
Rajakumara et al. (2012) reported the synthesis of TiO2NPs(62−74 nm\mathrm{TiO}_{2} \mathrm{NPs}(62-74 \mathrm{~nm}, oval and spherical) using Aspergillus flavus as a reducing and capping agent. The resulting NPs have antimicrobial activity against
Streptomyces aureus, E. coli, Klebsiella pneumoniae and B. subtilis. Rice straw as a lignocellulosic waste material has been used for production of TiO2NPs\mathrm{TiO}_{2} \mathrm{NPs} by a sol-gel method and further modification of the pore volume and size and decreased particle size enhanced the surface area which made TiO2\mathrm{TiO}_{2} NPs highly potential photocatalyst (Ramimoghadam et al. 2014). Anwar et al. (2010) used a sucrose ester-mediated hydrothermal processing route to produce TiO2NPs\mathrm{TiO}_{2} \mathrm{NPs}. Furthermore, they reported the changes in shape of NPs from needle to rod, rod to spherical by increase in temperature.
Bio-reduction activity of leaf extract of Catharanthus roseus resulted in synthesis of TiO2\mathrm{TiO}_{2} NPs of 25−110 nm25-110 \mathrm{~nm} size and irregular shape at 50∘C50^{\circ} \mathrm{C} (Velayutham et al. 2012). The produced NPs exhibited parasitic activity against sheep-biting lice Bovicola ovis and Hippobosca maculata. Highly stable and uniform TiO2NPs(100−150 nm)\mathrm{TiO}_{2} \mathrm{NPs}(100-150 \mathrm{~nm}) are produced by using 0.4 M of titanium tetraisopropoxide and Nyctanthes arbor-tristis leaf extract (Sundrarajan and Gowri 2011).
Copper (Cu)(\mathrm{Cu}) and copper oxide (CuO)(\mathrm{CuO})
Copper NPs with an average particle size less than 2 nm were prepared using non toxic L-ascorbic acid which acted as reducing agent and stabilizer (Xiong et al. 2011). Aloe leaf extract and copper sulfate (Cu2SO4)\left(\mathrm{Cu}_{2} \mathrm{SO}_{4}\right) solution under vigorous stirring at 130∘C130^{\circ} \mathrm{C} for 7 h
Table 2 Biological entities which synthesize pure metal nanoparticles with their size and shape and brief experiments
| Biological entities | Precursors, conditions | NPa\mathrm{NP}^{\mathrm{a}}, size and shape | Key aspects | Ref b ^{\text {b }} |
|---|---|---|---|---|
| Catharanthus roseus (plant) Source leaves | Pd(OAc)2 h\mathrm{Pd}(\mathrm{OAc}) 2 \mathrm{~h} at 60∘C60^{\circ} \mathrm{C} | Pd 38 nm , spherical | Effective in textile effluent remediation | Kalaiselvi et al. (2015) |
| Cocos nucifera (plant) | Pb(COOH)2\mathrm{Pb}(\mathrm{COOH})_{2} at 37∘C37^{\circ} \mathrm{C} | Pb 47 nm | Absorption of carcinogenic dye | Elango and Roopan (2015) |
| Croton sparsiflorus (plant) Source leaves | AgNO3\mathrm{AgNO}_{3} at 29∘C29^{\circ} \mathrm{C} | Ag,22−52 nm\mathrm{Ag}, 22-52 \mathrm{~nm}, spherical | Effective against S. aureus, E. coli, B. Subtilis. Dark brown colour indicated formation of NPs | Kathiravan et al. (2015) |
| Olive (plant) Source leaves | AgNO324 h\mathrm{AgNO}_{3} 24 \mathrm{~h} | Ag,20−25 nm\mathrm{Ag}, 20-25 \mathrm{~nm}, spherical | Effective against drug resistance bacterial isolates | Khalil et al. (2014) |
| Volvariella volvacea (fungus) | HAuCl4⋅3H2O\mathrm{HAuCl}_{4} \cdot 3 \mathrm{H}_{2} \mathrm{O} and AgNO3,2.5\mathrm{AgNO}_{3}, 2.5 and 6 h for Au and Ag respectively | Ag and Au, 20−150 nm20-150 \mathrm{~nm}, triangular, spherical, hexagonal | Au NPs are bound to proteins through free amino groups and silver NPs through the carboxylate group of the amino acid residues | Philip (2009a) |
| Honey Bee (animal) Source honey | HAuCl4,30\mathrm{HAuCl} 4,30 min at pH 3 | Au, Avg 15 nm | Use of animal product | Philip (2009b) |
| Dioscorea batatas (plant) Source rhizome | AgNO3 At 25 and 80∘C\begin{aligned} & \mathrm{AgNO}_{3} \\ & \text { At } 25 \text { and } 80^{\circ} \mathrm{C} \end{aligned} | Ag, flower, spherical | Antimicrobial activity against C. albicans and S. cerevisiae | Nagajyothi and Lee (2011) |
| Citrus (plant) Source peel | AgNO3,25\mathrm{AgNO}_{3}, 25 and 60 | Ag,35\mathrm{Ag}, 35 and 10 nm , Spherical | Effective against E. coli than S. aureus | Kaviyaa et al. (2011) |
| Sorghum (plant) Source bran powder | FeCl3\mathrm{FeCl}_{3} and AgNO3,1 h\mathrm{AgNO}_{3}, 1 \mathrm{~h} at 37∘C37^{\circ} \mathrm{C} | Fe and Ag, Avg 50 nm | Environmental remediation and treatment of hazardous waste | Njagi et al. (2011) |
| Eucalyptus (plant) Source leaves | FeSO4,At37∘C\mathrm{FeSO}_{4}, \mathrm{At} 37^{\circ} \mathrm{C} | Fe,20−80 nm\mathrm{Fe}, 20-80 \mathrm{~nm} | 71.7, 30.4 and 84.5% N,P84.5 \% \mathrm{~N}, \mathrm{P} and COD respectively were removed from contaminated water | Wang et al. (2014a) |
| Green tea and Eucalyptus (plants) Source leaves | FeSO430 min\mathrm{FeSO}_{4} 30 \mathrm{~min} at 37∘C37^{\circ} \mathrm{C} | Fe,20−80 nm\mathrm{Fe}, 20-80 \mathrm{~nm}, spherical | Remediation of nitrate contaminated sites | Wang et al. (2014b) |
| Sargassum muticum (algae) | FeCl36H2O90 min\mathrm{FeCl}_{3} 6 \mathrm{H}_{2} \mathrm{O} 90 \mathrm{~min} | Fe,Avg18 nm\mathrm{Fe}, \mathrm{Avg} 18 \mathrm{~nm}, cubic | Recycled and removed by magnetic device | Mahdavi et al. (2013) |
| Carica papaya (plant) Source leaves | FeCl36H2O\mathrm{FeCl}_{3} 6 \mathrm{H}_{2} \mathrm{O} Few min at 37∘C37^{\circ} \mathrm{C} | Fe,Avg33 nm\mathrm{Fe}, \mathrm{Avg} 33 \mathrm{~nm}, spherical | Rapid method. Black colour indicated formation of NPs a | Latha and Gowri (2014) |
| Streptomyces sp. (bacterium) | MnSO4\mathrm{MnSO}_{4} and ZnSO4\mathrm{ZnSO}_{4} 4 days at 35∘C35^{\circ} \mathrm{C} | Mn and Zn , 10−20 nm10-20 \mathrm{~nm} | Produced Mn and Zn NPs by same procedure | Waghmare et al. (2011) |
| Gloriosa superba (plant) Source leaves | RuCl320 min\mathrm{RuCl}_{3} 20 \mathrm{~min} at 100∘C100^{\circ} \mathrm{C} | Ru, Avg 36 nm | Effective against gram +ve than gram -ve bacteria | Gopinath et al. (2014) |
- a{ }^{a} Nanoparticles
b { }^{\text {b }} References ↩︎
resulted in synthesis of mono-disperse CuO NPs (Gunalan et al. 2012a, b). The particles has spherical shape and ranged in size from about 15−30 nm15-30 \mathrm{~nm}.
The CuO NPs (5-10 nm, spherical) have been prepared by using 1 ml plant extract and cupric nitrate Cu(NO3)2\mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2} in homogenous mixture at 400∘C400^{\circ} \mathrm{C} for 3−4 min3-4 \mathrm{~min} (Naikaa et al. 2015). The NPs possessed good antibacterial activity against pathogenic bacteria such as Staphylococcus aureus and Klebsiella aeroegenes. Aqueous solution of Cu2SO4\mathrm{Cu}_{2} \mathrm{SO}_{4} when treated with the Cassia alata flower extract produced stable CuO NPs (110-280 nm, spherical) within 45 min (Jayalakshmi and Yogamoorthi 2014). The leaf extract of Malva sylvestris has been used as a reducing agent in the extracellular synthesis of CuO NPs (5-10 nm, spherical) (Awwad et al. 2015). The particles had antimicrobial activity against both Gram-positive and Gram-negative bacteria.
Mixtures with various concentrations 1, 2 and 3 mM of CuCl2⋅H2O\mathrm{CuCl}_{2} \cdot \mathrm{H}_{2} \mathrm{O} and Gum karya at 10mg/ml10 \mathrm{mg} / \mathrm{ml} kept at 75∘C75^{\circ} \mathrm{C} at 250 rpm for 1 h in an orbital shaker produced CuO NPs of 4.8,5.54.8,5.5 and 7.8 nm size respectively (Vellora et al. 2013). It is evident that the increase in concentration of precursor enhances the particle size. Acharyulu et al. (2014) used a leaf extract of Phyllanthus amarus to produce CuO NPs ( 20 nm , spherical). The NPs were shown to be greater antibacterial activity against B. subtilis in comparison to rifampicin.
Zinc oxide (ZnO)
Extracellular synthesis of ZnO NPs (25-40 nm, spherical) is possible by reduction of aqueous Zn+\mathrm{Zn}^{+} with an extract of Aloe vera (Gunalan et al. 2011). The size of NPs varies with concentrations of leaf broth solution. Cassia auriculata flower extract was used for treatment of aqueous solution of Za(NO3)2\mathrm{Za}\left(\mathrm{NO}_{3}\right)_{2} to synthesize stable ZnO NPs with average size between 110 to 280 nm (Ramesh et al. 2014). The biocidal activity of green-synthesized ZnO NPs was higher against various pathogens as compared with ZnO NPs synthesized by a chemical method (Gunalan et al. 2012a, b).
Aeromonas hydrophila could synthesize ZnO NPs ( 57.7 nm , spherical) that exhibited antimicrobial activity against Pseudomonas aeruginosa and Aspergillus flavus (Jayaseelana et al. 2012). Senthilkumar and Sivakumar (2014) reported the green tea-mediated synthesis of ZnO NPs ( 16 nm ). These particles had
activities against Aspergillus flavus and Klebsiella pneumoniae. ZnO NPs (30-35 nm, spongy shape) have been synthesized using a leaf extract of Hibiscus rosa-sinesis at 100∘C100^{\circ} \mathrm{C} until a deep yellow paste developed (Devi and Gayathri 2014). The synthesis of manganese and zinc NPs using Streptomyces sps p (HBUN 17119) was reported by Waghmare et al. (2011).
Cerium oxide (CeO2)\left(\mathrm{CeO}_{2}\right)
Priya et al. (2014) used Aloe barbadensis gel and Ce(NO3)3⋅6H2O\mathrm{Ce}\left(\mathrm{NO}_{3}\right)_{3} \cdot 6 \mathrm{H}_{2} \mathrm{O} to produce uniformly spherically shaped CeO2\mathrm{CeO}_{2} NPs with an average size of 63.3 nm . Extract of Gloriosa superba leaf has been used to produce CeO2\mathrm{CeO}_{2} NPs (Arumugama et al. 2015). These NPs ( 5 nm , spherical) displayed excellent antibacterial properties. The samples exhibited blue green emission at 486 nm due to presence of an oxygen vacancy and oxygen interstitial defects. The toxicological behavior of NPs was due to small size, with uneven ridges and oxygen defects. Synthesis of cerium oxide NPs via food and their neurotoxicity effects was reported by Darroudi et al. (2014).
Khan and Ahmed (2013) reported fungal mediated biosynthesis of biomedically important CeO2\mathrm{CeO}_{2} NPs (12-20 nm, spherical). They established that the capping protein made the NPs water dispersible with clinical application for treatment of diseases by producing ROS. A simple one step, eco-friendly, bio-organic agarose polymer based synthesis of CeO2\mathrm{CeO}_{2} NPs ( 10 nm ) has been reported by Kargara et al. (2015). The authors observed that the NPs above 200∘C200^{\circ} \mathrm{C} possessed high homogeneity with the cubic fluorite structure and exhibited no significant cytotoxic effect on the L929 cell line at different concentrations, thus have viable applications in different fields of medicine.
Iron ( Fe ) and its oxides
The rapid biosynthesis of β\beta-iron oxide NPs ( <100 nm<100 \mathrm{~nm} ) was done by adding Eucalyptus globulus leaf extract in the aqueous solution of FeCl3\mathrm{FeCl}_{3} (Balamurugan 2014). In an another rapid single step, green synthesis of Fe and Ag NPs occurred using aqueous sorghum extracts as both reducing and capping agent (Njagi et al. 2011). The produced NPs effectively catalyzed H2O2\mathrm{H}_{2} \mathrm{O}_{2} degradation which made them useful for environmental
remediation and treatment of hazardous waste. FeCl3\mathrm{FeCl}_{3} and the extracts of different parts of plants were used to produce Fe NPs at 50−60∘C50-60^{\circ} \mathrm{C} (Shah et al. 2014). The authors reported that stem and leaf extracts of Calotropis procera did not produce NPs while the stem extract of Euphorbia produced NPs of minimum ranging 13−21 nm13-21 \mathrm{~nm} with spherical morphology.
Spherical Fe NPs were synthesized via a facile onestep green method using Eucalyptus leaf extract for treatment of eutrophic waste water (Wang et al. 2014a). They reported that 72%72 \% of total nitrogen, 30%30 \% of total phosphorus, 85%85 \% of chemical oxygen demand (COD) were removed respectively by the produced NPs. Thus NPs may play great role in remediation of waste water. In another experiment, Wang et al. (2014b) synthesized Fe NPs (20-80 nm, spherical) using extract of green tea and eucalyptus. Fe NPs were reactive towards nitrate in comparison to the traditional chemically prepared Fe3O4\mathrm{Fe}_{3} \mathrm{O}_{4} NPs. The biologically produced NPs showed significant in situ remediation of waste water especially in nitrate contaminated sites.
Machado et al. (2013) synthesized zero-valent iron NPs of spherical shape within size 10−30 nm10-30 \mathrm{~nm} using 1 ml of plant extract and 20μl20 \mu \mathrm{l} of an iron (III) solution. The better extractions may be obtained by leaves with low moisture content at 80∘C80^{\circ} \mathrm{C}. The aqueous extract of brown seaweed contains sulphated polysaccharides as reducing and stabilizer agent that have resulted in the synthesis of Fe3O4\mathrm{Fe}_{3} \mathrm{O}_{4} NPs (Mahdavi et al. 2013). The bioactivity of NPs ( 18±4 nm18 \pm 4 \mathrm{~nm}, cubic structure) against microbe was comparably higher than the particles synthesized via chemical method. The authors also observed that magnetic NPs can be removed or recycled in the medium using simple magnetic device.
In an elegant study, Balamurughan et al. (2014) reported the rapid biological synthesis of iron oxide NPs (20-47 nm, irregular) using leaves of Ocimum sanctum. The phenolic compounds and proteins present in extract were mainly responsible for reduction of ferrous ions. Latha and Gowri (2014) reported the use of an extract of Carica papaya leaves as an effective reductant for making Fe3O4\mathrm{Fe}_{3} \mathrm{O}_{4} NPs ( 33 nm , spherical) at normal room temperature. Synthesis of iron oxide NPs using aqueous extracts of monocotyledonous plant Hordeum, ( <30 nm<30 \mathrm{~nm}, unstable) and dicotyledonous plant Rumex,
(10-40 nm, highly stable) has been reported by Makarov et al. (2014).
Cadmium sulfide (CdS)
Cadmium sulfide (CdS) NPs (100-200 nm, spherical) were produced under ambient conditions using immobilized fungus Coriolus versicolor (Sanghi and Verma 2009). The fungus on exposure to toxic Cd2+\mathrm{Cd}^{2+} without an external source of sulphur, transformed toxic Cd to non-toxic CdS NPs and thus has great scope in remediation of toxic metals from soils. Conditions have also been standardized for the synthesis of semiconductor CdS NPs, 4.93 and 3.75 nm , using low-cost green and reproducible Lactobacillus sp. and Saccharomyces cerevisiae respectively (Prasad and Jha 2010). Immobilized Rhodobacter sphaeroides has been used to produce CdS NPs with average size of 2.3,6.82.3,6.8 and 36.8 nm at time interval of 36,42 and 48 h respectively (Bai et al. 2009). A simple green route for synthesis of CdS NPs (15-18 nm) using starch as a capping agent was successfully demonstrated (Wei et al. 2004).
Silver (Ag) and gold (Au)
The leaf extract of Croton sparsiflorus has been used to synthesis Ag NPs (22-52 nm, spherical shape) and the assemblies of spherical NPs seemed to be effective against S. aureus, E. coli, B. subtilis (Kathiravan et al. 2015). In another experiment a hot water extract of olive leaf was used for the production of Ag NPs (20-25 nm, spherical). The authors confirmed that the produced NPs possess good antibacterial activity against drug resistance bacterial isolates (Khalil et al. 2014).
Philip (2009a) reported the extracellular synthesis of Ag,Au\mathrm{Ag}, \mathrm{Au} and Au−Ag\mathrm{Au}-\mathrm{Ag} NPs (20-150 nm, triangular, spherical, and hexagonal) using water and an extract of Volvariella volvacea, an edible mushroom. Honeymediated biosynthesis of Au NPs was reported by Philip (2009b). A rhizome extract of Dioscorea batatas was used for production of Ag NPs (Nagajyothi and Lee 2011). The authors further reported that S. cerevisiae, Candida albicans were more susceptible to Ag NPs produced at room temperature than at 80∘C80^{\circ} \mathrm{C}. Ag NPs (62-74 nm, oval and spherical) were produced using citrus peel as a reducing and capping agents (Kaviyaa et al. 2011).
Palladium (Pd)
Palladium (Pd) NPs are of interest because of their catalytic properties and affinity for H2\mathrm{H}_{2}. Kanchana et al. (2010) reported the synthesis of Pd NPs using Solanum trilobatum. Catharanthus roseus leaf extract also has potential for the formation of Pd NPs because of phenolics responsible for reducing the Pd to zero valency (Kalaiselvi et al. 2015). The produced NPs are effective in dye degradation at pH 8 thus used in textile effluent remediation. Extracts from commercial products, like coffee and tea, were used in NPs synthesis. Pd and Ag NPs of 20−60 nm20-60 \mathrm{~nm} in size with cubic symmetry were synthesized from coffee and tea extract using PdCl2\mathrm{PdCl}_{2} and AgNO3\mathrm{AgNO}_{3} as precursor salt at room temperature (Nadagouda and Varma 2008). They suggested the method may be extended for other nobel metals such as Au and Pt.
Root extract of Euphorbia condylocarpa synthesized Pd/Fe3O4\mathrm{Pd} / \mathrm{Fe}_{3} \mathrm{O}_{4} NPs with an average size of 39 nm (Nasrollahzadeha et al. 2015a). The NPs were magnetically recoverable and served as a recyclable catalyst for phosphate-free Sonogashira-and Suzuki-coupling reactions. Nasrollahzadeha et al. (2015b) synthesized Pd NPs (2.5-14 nm, spherical) using a leaf extract of Hippophae rhamnoides. The authors explored that the produced NPs may be used as recyclable catalyst for Suzuki-coupling reactions. Pd NPs ( 15 nm ) were synthesized using a leaf extract of Glycine max (Petla et al. 2012).
Lead sulfide (PbS) and ruthenium (Ru)
Bacterial strains NS2 and NS6 have been used for the extracellular synthesis of lead sulfide (PbS) NPs (Singh and Naraa 2013). The authors demonstrated bioremediation technique that converted the toxic heavy metal Pb into less toxic PbS NPs. The methanolic extract of Cocos nucifera was employed as reducing and capping agent in the synthesis of Pb NPs that had an average diameter of 47 nm (Elango and Roopan 2015). The particles showed photocatalytic absorption of Malachite Green dye, a carcinogenic dye with a good antimicrobial activity against SS. aureus.
Gopinath et al. (2014) reported an eco-friendly approach for the synthesis of ruthenium NPs ( 36 nm ) using a leaf extract of Gloriosa superba. The NPs interference with the membrane especially gram
positive bacteria leads to cell death because of structural changes.
Characterization
Once the NPs are synthesized, their conformational details about shape, size, dispersity, homogeneity as well as surface morphology are determined by using various techniques. The common techniques of characterizing NPs are as follows: UV-Vis absorption spectroscopy, X-ray diffraction (XRD), Fourier transmission infrared (FTIR) spectroscopy, dynamic light scattering (DLS), energy dispersive X-ray analysis (EDAX), scanning electron microscopy (SEM), transmission electron microscopy (TEM), etc.
UV-Vis spectra were employed to examine the size and shape of NPs in aqueous suspension (Rajesh et al. 2009). Wavelengths from 300 to 800 nm are normally used for characterization of NPs ranging in size from about 2−100 nm2-100 \mathrm{~nm} (Feldheim and Foss 2002). UV-Vis spectra of the ZnO particles synthesized using Aloe vera extract exhibited strong UV absorption spectra with the absorption peak ranging from 358 to 375 nm due to its surface plasmon resonance (Gunalan et al. 2011).
The morphology and size of NPs are usually characterized by SEM and TEM (Schaffer et al. 2009). Electron microscopy analysis displayed ZnO NPs (25-55 nm), which is in agreement with the XRD analysis (Gunalan et al. 2011). SEM and TEM analysis of green synthesized carbon nanotubes were covered completely with polyaniline layers (Nguyen and Shim 2015). In TEM analysis, TiO2\mathrm{TiO}_{2} particles were agglomerated mostly spherical in shape in the range of 10−30 nm10-30 \mathrm{~nm}. Furthermore, the selected area electron diffraction (SAED) analysis indicated a crystalline shape (Dhandapani et al. 2012).
XRD gives information about translational symmetry, size and phase identification of metallic NPs (Sun et al. 2000). X-rays penetrate into the nanomaterials and the obtained diffraction pattern is compared with standards to get structural information. XRD peaks located at angles (2Ө) of 28.51,33.0628.51,33.06 and 47.42 corresponding to 111,200 and 220 planes and the standard diffraction peaks show the face-centre cubic phase of CeO2\mathrm{CeO}_{2} NPs (Arumugama et al. 2015). XRD study confirmed the presence of crystalline pattern of Pb NPs and the average particle size 47 nm using Scherer equation (Elango and Roopan 2015).
FTIR spectroscopy is used to determine the nature of functional groups or metabolites present on the surface of NPs which might be responsible for reduction and stabilization of NPs (Sankar et al. 2014). Functional group bands observed at 3450, 3266 and 2932 cm−12932 \mathrm{~cm}^{-1} have been assigned to stretching vibrations of the amines, O−H\mathrm{O}-\mathrm{H} stretching of alcohols and C−H\mathrm{C}-\mathrm{H} stretching of alkanes respectively for NPs using Aloe vera leaf extracts and the peaks in the region between 600 and 400 cm−1400 \mathrm{~cm}^{-1} are allotted to ZnO (Gunalan et al. 2011). The FTIR spectrum of Ag NPs synthesized using Solanum torvum leaf extract exhibited peaks at 1648,1535,14501648,1535,1450 and 1019 cm−11019 \mathrm{~cm}^{-1} and the peak at 1450 cm−11450 \mathrm{~cm}^{-1} of carboxylate ions were said to be responsible for stabilizing the Ag NPs (Govindaraju et al. 2010).
The DLS and EDAX are exercised to analyse the size distribution dispersed in liquid and the elemental constituents of NPs respectively (Jiang et al. 2009; Strasser et al. 2010).
Applications of green nanotechnology
In the last decade, there has been a dramatic increase in scientific publications in the field of nanotechnology. Green-synthesized nanomaterials play significant roles in application of nanotechnology to diverse fields. Green nanotechnology refers to the formation of green nano-products and use of these products to achieve sustainable development.
Green synthesized NPs play significant roles in medicines, clinical applications and in vitro diagnostic applications (Gunalan et al. 2012a, b; Khan and Ahmad 2013; Jayalakshmi and Yogamoorthi 2014; Arumugama et al. 2015; Kargara et al. 2015). NPs synthesized via green methods show excellent antibacterial effects (Jayaseelana et al. 2012; Rajakumara et al. 2012; Mahdavi et al. 2013; Acharyulu et al. 2014; Gopinath et al. 2014; Naikaa et al. 2015; Awwad et al. 2015), antifungal effects (Sanghi and Verma 2009) and anti-parasitic activity (Velayutham et al. 2012).
Green nanoparticles have been exploited. Gusseme et al. (2010) reported the use of biogenic silver produced using Lactobacillus fermentum for the removal of viruses from drinking water. Aquapure and QSI-Nano, are commercially available for home-water purification systems containing silver
as a disinfectant (Maynard 2007). Magnetotactic bacteria (MTB) NPs are utilized for gene delivery and PEI-associated MTB-NPs to deliver β\beta-galactosidase plasmids both in vitro and in vivo (Xie et al. 2009). Co-transfection of small interfering RNA (siRNA) with quantum dots by standard transfection techniques has led the formation of photostable fluorescent NPs that help in tracking the delivery of nucleic acid, the degree of transfection in cells and in purifying homogeneously silenced sub populations (Chen et al. 2005). NPs in the size range of 1−100 nm1-100 \mathrm{~nm} readily bind with the HIV-1 virus on gp120 glycoprotein knobs. This specific interaction of NPs inhibits the virus from binding to host cells, thus help prevent and control of HIV infection (Elechiguerra et al. 2005).
NPs can cause cell wall damage, membrane damage or produce free radicals resulting in induction of oxidative, DNA or electron transport chain damage consequently leads to bacterial death (Chaloupka et al. 2010; Gopinath et al. 2014). Schematic representation of cellular uptake of NPs and the mechanism of toxicity of NPs against bacteria is presented in Fig. 3.
Nanomaterials or their products are useful in environmental remediation (Njagi et al. 2011). In these greener routes, organisms or their products or NPs clean hazardous waste sites (Kalaiselvi et al. 2015; Elango and Roopan 2015) and treat pollutants (Sankar et al. 2013; Singh and Naraa 2013). Green nanomaterials have a wide scope in the treatment of surface water, groundwater and wastewater contaminated by toxic metal ions, organic and inorganic solutes and microorganisms (Dhandapani et al. 2012). Self-cleaning nanoscale surface coatings may eliminate many cleaning chemicals used in regular maintenance routines (Elango and Roopan 2015). Fe NPs are of considerable interest because of their rapidly developing applications for disinfection of water and remediation of heavy metals from soils (Wang et al. 2014a, b; Mahdavi et al. 2013).
NPs are alternatives to pesticides in control and management of plant disease (Khot et al. 2012; Singh et al. 2015a, b) and they also act as effective fertilizers (Kottegoda et al. 2011) which were eco-friendly and increase crop production. Magnetite (Fe3O4)/\left(\mathrm{Fe}_{3} \mathrm{O}_{4}\right) / greigite (Fe3 S4)\left(\mathrm{Fe}_{3} \mathrm{~S}_{4}\right) and siliceous material produced using bacteria and diatoms respectively are successfully used in optical coatings for solar energy applications and as

Fig. 3 Schematic representation of cellular uptake of nanoparticles and the mechanism of particle induced toxicity against bacteria
ion insertion materials for electrical battery applications (Joerger et al. 1999) Nanoscale catalysts form chemical reactions more efficient and less wasteful (Nasrollahzadeha et al. 2015b).
Conclusions and future prospective
Production of NPs using extracts from natural substances is emerging as an important area in
nanotechnology. The use of natural resources for production of NPs is sustainable, eco-friendly, inexpensive and free of chemical contaminants for biological and medical applications where purity of NPs is of major concern. Useful and common nanomaterials can be produced easily on large scale. The biological methods do not need harsh or toxic chemicals. The waste products of plant extracts are non toxic and easier to dispose off. Furthermore, NPs synthesized via green route are more stable and effective in comparison with those produced by physico-chemical methods.
The majority of greener synthetic efforts reported earlier are dedicated to Ag and Au NPs, which may be due to their importance in disinfection science. This report devoted to several other metals and its oxides NPs viz. Fe,Pd,Ru,PbS,CdS,CuO,CeO2,TiO2\mathrm{Fe}, \mathrm{Pd}, \mathrm{Ru}, \mathrm{PbS}, \mathrm{CdS}, \mathrm{CuO}, \mathrm{CeO}_{2}, \mathrm{TiO}_{2}, and ZnO NPs synthesized by biological methods which have imperative roles in human welfare.
A considerable number of efforts have been taken in order to obtain secondary metabolites from the extract of natural products which may act as reducing, stabilizing and capping agents in the synthesis process of nanomaterials. Capping and stabilizing agents present in biological entities act as growth terminator and inhibits agglomeration processes and thus enhances the stability and persistence of NPs. The nature of biological entities in different concentrations with combination of organic reducing agents influences the size and shape of NPs.
The most of these investigations have been carried out in research laboratories in small scale but researchers are engaged to explore the potential and application of NPs at large scale in agricultural field, environment, health science and many more to fulfill the future demands of growing population of world and to provide best service for human welfare.
Acknowledgments The authors are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, University Grant Commission (UGC), New Delhi and University of Allahabad, Allahabad, India for providing financial assistance to Imtiyaz Hussain.
References
Acharyulu NPS, Dubey RS, Swaminadham V, Kalyani RL, Kollu P, Pammi SVN (2014) Green synthesis of CuO nanoparticles using Phyllanthus amarus leaf extract and
their antibacterial activity against multidrug resistance bacteria. Int J Eng Res Technol 3:639-641
Aenishanslins NAO, Saona LA, Durán-Toro VM, Monrás JP, Bravo DM, Donoso JMP (2014) Use of titanium dioxide nanoparticles biosynthesized by Bacillus mycoides in quantum dot sensitized solar cells. Microb Cell Factories 13:90
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R et al (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf 28:313-318
Anwar NS, Kassim A, Lim HN, Zakarya SA, Huang NM (2010) Synthesis of titanium dioxide nanoparticles via sucrose ester micelle-mediated hydrothermal processing route. Sains Malays 39:261-265
Aromal SA, Vidhu VK, Philip D (2012) Green synthesis of welldispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim Acta Part A 85:99-104
Arumugama A, Karthikeyan C, Hameed ASH, Gopinath K, Gowri S, Karthika V (2015) Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng 49:408-415
Awwad AM, Albiss BA, Salem NM (2015) Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract. SMU Med J 2:91-101
Bai H, Zhang Z, Guo Y, Jia W (2009) Biological synthesis of size-controlled cadmium sulfide nanoparticles using immobilized Rhodobacter sphaeroides. Nanoscale Res Lett 4:717-723
Balamurugan M (2014) Synthesis of iron oxide nanoparticles by using Eucalyptus globulus plant extract. Surf Sci Nanotechnol 12:363-367
Balamuraghan MG, Mohanraj S, Kodhaiyolii S, Pugalenthi V (2014) Ocimum sanctum leaf extract mediated green synthesis of iron oxide nanoparticles. Spectroscopic and microscopic studies 4:201-204
Bharde A, Wani A, Shouche Y, Pattayil A, Bhagavatula L, Sastry M (2005) Bacterial aerobic synthesis of nanocrystalline magnetite. JACS 127:9326-9327
Botes M, Cloete TE (2010) The potential of nanofibers and nanobiocides in water purification. Crit Rev Microbiol 36:68-81
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation nanoproduct in biomedical applications. Trends Biotechnol 28:580-588
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577-583
Chen AA, Derfus AM, Khetani SR, Bhatia SN (2005) Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res 33:1-8
Dameron CT, Reese RN, Mehra RK, Kortan AR, Carroll PJ, Steigerwald ML et al (1989) Biosynthesis of cadmium sulfide quantum semiconductor crystallites. Lett Nat 338:596-597
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293-346
Darroudi M, Hoseini SJ, Oskuee RK, Hosseini HA, Gholami L, Gerayli S (2014) Food-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effects. Ceram Int 40:7425-7430
Devi RS, Gayathri R (2014) Green Synthesis of zinc oxide nanoparticles by using Hibiscus rosa-sinensis. Int J Curr Eng Technol 4:2444-2446
Dhandapani P, Maruthamuthu S, Rajagopal G (2012) Bio-mediated synthesis of TiO2\mathrm{TiO}_{2} nanoparticles and its photocatalytic effect on aquatic biofilm. J Photochem Photobiol, B 110:43-49
Dhillon GS, Brar SK, Kaur S, Verma M (2012) Green approach for nanoparticle biosynthesis by fungi current trends and applications. Crit Rev Biotechnol 32:49-73
Duran N, Marcato PD, Durán M, Yadav A, Gade A, Rai M (2011) Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi and plants. Appl Microbiol Biotechnol 90:1609-1624
Dwivedi AD, Gopal K (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A 369:27-33
Elango G, Roopan SM (2015) Green synthesis, spectroscopic investigation and photocatalytic activity of lead nanoparticles. Spectrochim Acta Part A 139:367-373
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH et al (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 3:1-10
Feldheim DL, Foss CA (2002) Metal nanoparticles: synthesis, characterization, and applications. CRC Press, Boca Raton, p 338
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132-140
Gopinath K, Shanmugam VK, Gowri S, Senthilkumar V, Kumaresan S, Arumugam A (2014) Antibacterial activity of ruthenium nanoparticles synthesized using Gloriosa superba L. leaf extract. J Nanostruct Chem 4:83
Govindaraju K, Basha SK, Kumar VG, Singaravelu G (2008) Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis). J Mater Sci 43:5115-5122
Govindaraju K, Tamilselvan S, Kiruthiga V, Singaravelu G (2010) Biogenic silver nanoparticles by Solanum torvum and their promising antimicrobial activity. J Biopest 3:349-399
Gunalan S, Sivaraj R, Venckatesh R (2011) Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: structure and optical properties. Mater Res Bull 46:2560-2566
Gunalan S, Sivaraj R, Rajendran V (2012a) Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci 22:693-700
Gunalan S, Sivaraj R, Venckatesh R (2012b) Aloe barbadensis miller mediated green synthesis of mono-disperse copper oxide nanoparticles optical properties. Spectrochim Acta Part A 97:1140-1144
Gusseme DB, Sintubin L, Baert L, Thibo E, Hennebel T, Vermeulen G, Uyttendaele M, Verstraete W, Boon N (2010) Biogenic silver for disinfection of water contaminated with viruses. Appl Environ Microbiol 76:1082-1087
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X et al (2007) Biosynthesis of silver and gold nanoparticles by novel
sundried Cinnamomum camphora leaf. Nanotechnology 18:105104-105115
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638-2650
Jayalakshmi and Yogamoorthi A (2014) Green synthesis of copper oxide nanoparticles using aqueous extract of flowers of Cassia alata and particles characterisation. Int J Nanomat Biostruct 4:66-71
Jayaseelana C, Rahumana AA, Kirthi AV, Marimuthua S, Santhoshkumara T, Bagavana A et al (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochimica Acta Part A 90:78-84
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77-89
Joerger R, Klaus T, Olsson E, Granqvist CG (1999) Spectrally selective solar absorber coatings prepared by a biomimetic technique. Proc Soc Photo-Opt Instrum Eng 3789:2-7
Joglekar S, Kodam K, Dhaygude M, Hudlikar M (2011) Novel route for rapid biosynthesis of lead nanoparticles using aqueous extract of Jatropha curcas L. latex. Mater Lett 65:3170−317265: 3170-3172
Kalaiselvi A, Roopan SM, Madhumitha G, Ramalingam C, Elango G (2015) Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim Acta Part A 135:116-119
Kanchana A, Devarajan S, Ayyappan SR (2010) Green synthesis and characterization of palladium nanoparticles and its conjugates from Solanum trilobatum leaf extract. NanoMicro Lett 2:169-176
Kargara H, Ghasemi F, Darroudid M (2015) Bioorganic poly-mer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceram Int 41:1589-1594
Kathiravan V, Ravi S, Ashokkumar S, Velmurugan S, Elumalai K, Khatiwada CP (2015) Green synthesis of silver nanoparticles using Croton sparsiflorus morong leaf extract and their antibacterial and antifungal activities. Spectrochim Acta Part A 139:200-205
Kaushik N, Thakkar MS, Snehit S, Mhatre MS, Rasesh Y, Parikh MS (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6:257-262
Kaviyaa S, Santhanalakshmia J, Viswanathanb B, Muthumaryc J, Srinivasanc K (2011) Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta Part A 79:594-598
Khalil MMH, Ismail EH, Baghdady KZE, Mohamed D (2014) Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem 7:1131-1139
Khan SA, Ahmad A (2013) Fungus mediated synthesis of biomedically important cerium oxide nanoparticles. Mater Res Bull 48:4134-4138
Kharissova OV, Dias HVR, Kharisov BI, Perez BO, Victor M, Perez J (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31:240-248
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64-70
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279-306
Kottegoda N, Munaweera I, Madusanka N, Karunaratne V (2011) A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr Sci 101:73-78
Kubik B, Sugisaka M (2002) From molecular biology to nanotechnology and nanomedicine. Biosystems 65:123-138
Latha N, Gowri M (2014) Bio Synthesis and Characterisation of Fe3O4\mathrm{Fe}_{3} \mathrm{O}_{4} nanoparticles using Caricaya papaya leaves extract. International J Sci Res 3:1551-1556
Lee HJ, Lee G, Jang NR, Yun JM, Song JY, Kim BS (2011) Biological synthesis of copper nanoparticles using plant extract. Nanotechnology 1:371-374
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomate 2011:1-16
Lloyd JR, Byrne JM, Coker VS (2011) Biotechnological synthesis of functional nanomaterials. Curr Opin Biotechnol 22:509-515
Luangpipat T, Beattie IR, Chisti Y, Haverkamp RG (2011) Gold nanoparticles produced in a microalga. J Nanopart Res 13:6439-6445
Machado S, Pinto SL, Grosso JP, Nouws HPA, Albergaria JT, Matos CD (2013) Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ 445−446:1−8445-446: 1-8
Mahdavi M, Namvar F, Ahmad MB, Mohamad R (2013) Green Biosynthesis and characterization of magnetic iron oxide (Fe3O4)\left(\mathrm{Fe}_{3} \mathrm{O}_{4}\right) nanoparticles using seaweed Sargassum muticum aqueous extract. Molecules 18:5954-5964
Makarov VV, Makarova SS, Love AJ, Sinitsyna OV, Dudnik AO, Yaminsky IV et al (2014) Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants. Langmuir 30:5982-5988
Maynard AD (2007) Nanotechnologies: overview and issues. In: Simeonova PP, Opopol N, Luster MI (eds) Nanotechnol-ogy-toxicological issues and environmental safety. Springer, Dordrecht, pp 1-14
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31:346-356
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507-517
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI (2001) Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix. A novel biological approach to nanoparticle synthesis. Nano Lett 1:515-519
Nadagouda MN, Varma RS (2008) Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem 10:859-862
Nagajyothi PC, Lee KD (2011) Synthesis of plant-mediated silver nanoparticles using Dioscorea batatas rhizome extract and evaluation of their antimicrobial activities. J Nanomater
Naikaa HR, Lingarajua K, Manjunathb K, Kumar D, Nagarajuc G, Sureshd D, Nagabhushanae H (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9:7-12
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1-13
Nasrollahzadeha M, Sajadib SM, Maham M (2015a) Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J Mol Catal A 396:297-303
Nasrollahzadeha M, Sajadib SM, Vartoonia AR, Khalajc M (2015b) Green synthesis of Pd/Fe3O4\mathrm{Pd} / \mathrm{Fe}_{3} \mathrm{O}_{4} nanoparticles using Euphorbia condylocarpa M. bieb root extract and their catalytic applications as magnetically recoverable and stable recyclable catalysts for the phosphine-free Sonogashira and Suzuki coupling reactions. J Mol Catal A 396:31-39
Nguyen VH and Shim JJ (2015) Green synthesis and characterization of carbon nanotubes/polyaniline nanocomposites. J Spectrosc
Njagi HH, Stafford L, Genuino H, Galindo HM, Collins JB et al (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts Eric C. Langmuir 27:264-271
Petla RK, Vivekanandhan S, Misra M, Mohanty AK, Satyanarayana N (2012) Soybean (Glycine Max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 3:14-19
Philip D (2009a) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochimica Acta Part A 73:374-381
Philip D (2009b) Honey mediated green synthesis of gold nanoparticles. Spectrochimica Acta Part A 73:650-653
Prasad K, Jha AK (2010) Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J Colloid Interface Sci 342:68-72
Priya GS, Kanneganti A, Kumar KA, Rao KV, Bykkam S (2014) Biosynthesis of Cerium oxide nanoparticles using Aloe barbadensis miller gel. Int J Sci Res Publ 4:2250-3153
Rajakumar G, Rahuman AA, Priyamvada B, Khanna VG, Kumar DK, Sujin PJ (2012) Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater Lett 68:115-117
Rajakumara G, Rahumana AA, Roopan SM, Khannac VG, Elangoa G, Kamaraja C et al (2012) Fungus-mediated biosynthesis and characterization of TiO2\mathrm{TiO}_{2} nanoparticles and their activity against pathogenic bacteria. Spectrochimica Acta Part A 9:123-129
Rajesh WR, Jaya RL, Niranjan SK, Vijay DM, Sahebrao BK (2009) Phytosynthesis of silver nanoparticle using Gliricidia sepium. Curr Nanosci 5:117-122
Ramesh P, Rajendran A, Shisundaram MM (2014) Green synthesis of zinc oxide nanoparticles using flower extract cassia. J Nanosci Nanotechnol 2:41-45
Ramimoghadam D, Bagheri S, Bee S, Hamid A (2014) Biotemplated synthesis of anatase titanium dioxide nanoparticles via lignocellulosic waste material. BioMed Res Int
Sanghi R, Verma P (2009) A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus. Chem Eng J 155:886-891
Sankar R, Rizwana K, Shivashangari KS, Ravikumar V (2014) Ultra-rapid photocatalytic activity of Azadirachta indica
engineered colloidal titanium dioxide nanoparticles. Appl Nanosci 5:731-736
Schaffer B, Hohenester U, Trugler A, Hofer F (2009) Highresolution surface plasmon imaging of gold nanoparticles by energy-filtered transmission electron microscopy. Phys Rev B 79:0414011-0414014
Senthilkumar SR, Sivakumar T (2014) Green tea (Camellia sinensis) mediated synthesis of zinc oxide ( ZnO ) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci 6:461-465
Shah S, Dasgupta S, Chakraborty M, Vadakkekara R, Hajoori M (2014) Green synthesis of iron nanoparticles using plant extracts. Int J Biol Pharm Res 5:549-552
Singh N, Naraa S (2013) Biological synthesis and characterization of lead sulfide nanoparticles using bacterial isolates from heavy metal rich sites. Int J Agric Food Sci Technol 4:16−234: 16-23
Singh SC, Mishra SK, Srivastava RK, Gopal R (2010) Optical properties of selenium quantum dots produced with laser irradiation of water suspended Se nanoparticles. J Phys Chem 114:17374-17384
Singh A, Singh NB, Hussain I, Singh H, Singh SC (2015a) Plantnanoparticle interaction: an approach to improve agricultural practices and plant productivity. Int J Pharm Sci Inven 4:25−404: 25-40
Singh NB, Singh A, Hussain I, Singh H, Singh SC (2015b) Synthesis, characterization and application of ruthenium oxide nanoparticles on growth and metabolism of Brassica oleracea L. Adv Sci Lett 21:2635-2640
Sintubin L, Windt DW, Dick J, Mast J, Vander HD, Verstraete W, Boon N (2009) Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol 84:741-749
Sintubin L, Gusseme DB, Meeren VP, Pycke BFG, Verstraete W, Boon N (2011) The antibacterial activity of biogenic silver and its mode of action. Appl Microbiol Biotechnol 91:153-162
Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C (2010) Lattice-strain control of the activity in de alloyed coreshell fuel cell catalysts. Nat Chem 2:454-460
Sun S, Murray C, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287:1989-1992
Sundrarajan M, Gowri S (2011) Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 8:447-451
Sweeney RY, Mao C, Gao X, Burt JL, Belcher AM, Georgiou G et al (2004) Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem Biol 11:1553-1559
Torresdy JLG, Gomez E, Videa JRP, Parsons JG, Troiani H, Yacaman JM (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:13571361
Velayutham K, Rahuman AA, Rajakumar G, Santhoshkumar T, Marimuthu S, Jayaseelan C et al (2012) Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosea maculata and Bovicola ovis. Parasitol Res 111:2329-2337
Vellora V, Padil T, Černík M (2013) Green synthesis of copper oxide nanoparticles using Gum karaya as a biotemplate and their antibacterial application. Int J Nanomed 8:889-898
Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH (2006) Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf B Biointerfaces 53:55-59
Waghmare SS, Deshmukh AM, Kulkarni SW, Oswaldo LA (2011) Biosynthesis and characterization of manganese and zinc nanoparticles. Univers J Environ Res Technol 1:64-69
Wang XW, Zhang L, Ma CL, Song RY, Hou HB, Li DL (2009) Enrichment and separation of silver from waste solutions by metal ion imprinted membrane. Hydrometal 100:82-86
Wang T, Lin J, Chen Z, Megharaj M, Naidu R (2014a) Green synthesis of Fe nanoparticles using Eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466−467:210−213466-467: 210-213
Wang T, Lin J, Chen Z, Megharaj M, Naidu R (2014b) Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J Clean Prod 83:413-419
Wei Q, Kang SZ, Mu J (2004) Green synthesis of starch capped CdS nanoparticles. Colloids Surf A 247:125-127
Xie J, Chen K, Chen X (2009) Production, modification and bioapplications of magnetic nanoparticles gestated by magnetotactic bacteria. Nano Res 2:261-278
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem 13:900-904
Zharov VP, Kim JW, Curiel DT, Everts M (2005) Nanomed. Self-assembling nanoclusters in living systems: application for integrated photothermal nanodiagnostics and nanotherapy. Nanotechnol. Biol Med 1:326-345