Cobalt | AMERICAN ELEMENTS® (original) (raw)
About Cobalt
Much like the “kupfernickel”--devil’s copper--ores that had been so named for failing to produce the copper they were thought to contain, cobalt’s ores were frustrating to miners. Not only did they fail to yield useful metals, these ores additionally produced toxic arsenic oxide when smelted. The ores were therefore named after kobolds--goblins that German miners frequently blamed for mining mishaps. When, around 1935, the Swedish chemist Georg Brandt succeeded in isolating cobalt metal, he named his newly discovered element for its ore.
In addition to discovering cobalt, Brandt demonstrated that cobalt compounds had been unknowingly used to provide coloring in smalt, a blue glass, since the middle ages. In the late eighteenth and early nineteenth century, green and blue pigments based on cobalt were developed and came into widespread use for coloring ceramics, jewelry, and paint. These applications for cobalt remain relevant in modern times, but today cobalt is used most often in metallic form.
The majority of cobalt consumed in the United States is used in the production of superalloys, metal formulations which most often find use in arenas where resistance to extreme conditions are required, such as in the components of jet engines or high-speed drill bits. Superalloys are also sometimes used in biomedical implants such as hip replacements, though these implants must be monitored for damage, as metal nanoparticles produced by wear are easily absorbed by and distributed through the body. Cobalt found in cobalamin, also known as vitamin B12, is an essential nutrient, but excess free cobalt ions in the body have toxic effects.
Cobalt is also used in other alloy applications. It is found in both Alnico and samarium-cobalt magnets, both of which are used widely in industry. Cobalt is also found in combination with primary electrode metals in lithium ion, nickel-cadmium, and nickel metal hydride batteries. Cobalt’s attractive appearance, extreme hardness, and resistance to oxidation lend it to use as a metal for plating of other materials, either alone or as the base for further coatings such as porcelain enamels. Platinum used in jewelry making contains five percent cobalt, as this produces an alloy that is suitable for highly detailed casting.
There are two other major uses for cobalt in industry. The first is as a catalyst: cobalt compounds are used industrially to produce polymer precursors, remove sulfurous impurities from petroleum, and improve adhesion of steel to rubber for the production of steel-belted tires. Additionally, cobalt catalysts are added as drying agents to paints and varnishes, and used in a variety of other chemical processes, on both industrial and lab-scales. The second other major use is as a binder in cemented carbides, extremely hard materials used in machining metals such as steel.
Finally, cobalt radioisotopes serve a few notable functions. Cobalt-60 is a radioactive isotope used to produce gamma rays for sterilizing food and medical supplies and for use in both medical radiotherapy and the in the production of industrial radiographs. Cobalt-57 is used as a tracer in medical imaging, primarily for observing vitamin B12 uptake.
The main cobalt ores are cobaltite, erythrite, glaucodot, and skutterudite, all of which are exploited commercially, but a substantial amount of the metal is also obtained from processing byproducts of copper and nickel mining. Cobalt catalysts and cobalt-alloy scrap may also be recycled to recover high purity cobalt.
Products
- [1,1'-Bis(diphenylphosphino)ferrocene]dichlorocobalt(II)
- [1,2-Bis(diphenylphosphino)ethane]dichlorocobalt(II)
- (1R,2R)-(-)-1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicylidene)cobalt(II)
- (3,3-Dimethyl-1-butyne)dicobalt Hexacarbonyl
- 5,10,15,20-Tetrakis(4-methoxyphenyl)-21H,23H-porphine cobalt(II)
- 5,10,15,20-Tetraphenyl-21H,23H-porphine Cobalt(II)
- Bis(2,2,6,6-tetramethyl-3,5-heptanedionato)cobalt(II)
- Bis(cyclopentadienyl)cobalt(II)
- Bis(cyclopentadienyl)cobalt(III) Hexafluorophosphate
- Bis(ethylcyclopentadienyl)cobalt(II)
- Bis(ethylcyclopentadienyl)cobalt(III) Hexafluorophosphate
- Bis(N,N'-di-i-propylacetamidinato)cobalt(II)
- Bis(N-t-butyl-N'-ethylpropanimidamidato)cobalt(II)
- Bis(pentamethylcyclopentadienyl)cobalt(II)
- Bis(pentamethylcyclopentadienyl)cobalt(III) Hexafluorophosphate
- Bis(salicylaldehyde)cobalt(II) Dihydrate
- Bis(salicylideniminato-3-propyl)methylaminocobalt(II)
- (Carbomethoxycyclopentadiene)(tetra phenylcyclobutadiene)cobalt(I)
- Chloro(pyridine)bis(dimethylglyoximato) cobalt(III)
- Chlorotris(triphenylphosphine)cobalt(I)
- Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
- Cobalt(II) 1,8,15,22-tetra(amino)phthalocyanine
- Cobalt(II) 2,3-naphthalocyanine
- Cobalt(II) 2,9,16,23-tetra(amino)phthalocyanine
- Cobalt 2-Ethylhexanoate
- Cobalt(II) 2-Ethylhexanoate Solution
- Cobalt(II) 2-Methoxyethoxide
- Cobalt(II) Acetylacetonate
- Cobalt(II) Acetylacetonate Hydrate
- Cobalt(III) Acetylacetonate
- Cobalt(II) Benzoylacetonate
- Cobalt Bis(trifluoromethylsulfonyl)imide
- Cobalt Carbonyl
- Cobalt(II) Chloride Tetrahydrofuran Complex
- Cobalt Citrate
- Cobalt Citrate Dihydrate
- Cobalt(II) Cyclohexanebutyrate
- Cobalt(II) Dibromo(1,2-dimethoxyethane)
- Cobalt Gluconate
- Cobalt Glycinate
- Cobalt Glycine
- Cobalt(II) Hexafluoroacetylacetonate Hydrate
- Cobalt Isopropoxide
- Cobalt Naphthenate
- Cobalt Neodecanoate
- Cobalt Octoate
- Cobalt(II) Oxo Pivalate
- Cobalt(II) Phthalocyanine
- Cobalt Potassium Citrate
- Cobalt(III) Sepulchrate Trichloride
- Cobalt Tricarbonyl Nitrosyl
- Cobalt(II) Trifluoroacetylacetonate Hydrate
- Cobalt Trifluoromethanesulfonate
- Cobalt Tris(2,2,6,6-tetramethyl-3,5-heptanedionate)
- Cyclopentadienyl(dimethyl fumarate)(triethyl phosphite)cobalt(I)
- Dicarbonylcyclopentadienyl Cobalt(I)
- Dichlorobis(triphenylphosphine)cobalt(II)
- FK 102 Co(II) PF6 Salt
- FK 102 Co(III) PF6 Salt
- FK 102 Co(II) TFSI Salt
- FK 102 Co(III) TFSI Salt
- FK 209 Co(II) PF6 Salt
- FK 209 Co(III) PF6 Salt
- FK 209 Co(II) TFSI Salt
- FK 209 Co(III) TFSI Salt
- FK 269 Co(II) PF6 Salt
- FK 269 Co(III) PF6 Salt
- FK 269 Co(II) TFSI Salt
- FK 269 Co(III) TFSI Salt
- [N,N'-Bis(3,5-di-tert-butylsalicylidene) -1,2-phenylenediaminochromium-di-THF]tetracarbonylcobaltate
- N,N'-Bis[3-tert-butyl-5-(heptadecafluorooctyl)salicylidene
- N,N'-Bis(salicylidene)-1,2-phenylenediaminocobalt(II) monohydrate
- N,N'-Bis(salicylidene)ethylenediaminocobalt(II)
- N,N'-Bis(salicylidene)ethylenediaminocobalt(II) Hydrate
- Protoporphyrin IX Cobalt Chloride
- Sodium Cobalticarborane
- Tetracobalt Dodecacarbonyl
- trans-Dichlorobis(ethylenediamine)cobalt(III) Chloride
- trans-Dinitrobis(ethylenediamine)cobalt(III) Nitrate
- Tris(1,10-phenanthroline)cobalt(II) Bis(hexafluorophosphate)
- Tris(1,10-phenanthroline)cobalt(III) Tris(hexafluorophosphate)
- Tris(ethylenediamine)cobalt(III) Chloride Dihydrate
- Tris(ethylenediamine)cobalt(III) Chloride Trihydrate
- Tris(ethylenediamine)cobalt(III) Nitrate
- ZIF-67, 2-methylimidazole Cobalt Salt
Cobalt is often alloyed with iron, nickel and other metals to make Alnico, an alloy of unusual magnetic strength with many important uses. Samarium-cobalt, for instance, is one of the highest strength magnet alloys known. Cobalt compounds are used to produce a brilliant and permanent blue color in ceramic glazes, glass,  pottery, tiles, and enamels. Co-60, a commercially important radioisotope, is useful as a radioactive tracer and gamma ray source.
pottery, tiles, and enamels. Co-60, a commercially important radioisotope, is useful as a radioactive tracer and gamma ray source.  Cobalt is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Cobalt nanoparticles and nanopowders are also available. Cobalt oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Cobalt fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cobalt is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Cobalt is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Cobalt nanoparticles and nanopowders are also available. Cobalt oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Cobalt fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cobalt is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Cobalt Properties
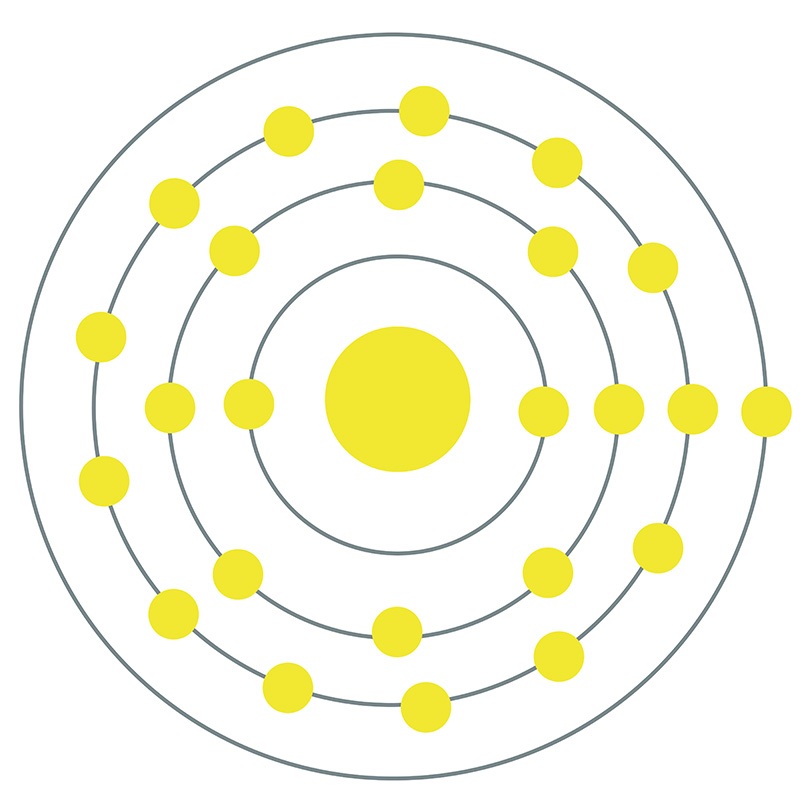
 Cobalt is a Block D, Group 9, Period 4 element. The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar] 3d7 4s2.
Cobalt is a Block D, Group 9, Period 4 element. The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar] 3d7 4s2.  The cobalt atom has a radius of 125.3.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-48-4, cobalt has a
The cobalt atom has a radius of 125.3.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-48-4, cobalt has a  lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores, but is not found free in nature. Cobalt was first discovered by George Brandt in 1732. The origin of the word Cobalt comes from the German word 'Kobalt or Kobold' which translates as "goblin", "elf" or "evil spirit".
lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores, but is not found free in nature. Cobalt was first discovered by George Brandt in 1732. The origin of the word Cobalt comes from the German word 'Kobalt or Kobold' which translates as "goblin", "elf" or "evil spirit".
| Symbol: | Co |
|---|---|
| Atomic Number: | 27 |
| Atomic Weight: | 58.9332 |
| Element Category: | transition metal |
| Group, Period, Block: | 9, 4, d |
| Color: | lustrous, metallic, grayish tinge /bluish-white |
| Other Names: | Cobaltum, Cobalto, Kobalt, Kobolt |
| Melting Point: | 1495.0 °C, 2723.0 °F, 1768.15 K |
|---|---|
| Boiling Point: | 2870.0 °C, 5198.0 °F, 3143.15 K |
| Density: | 8.9 gm/cm3 |
| Liquid Density @ Melting Point: | 7.75 g·cm3 |
| Density @ 20°C: | 8.90 g/cm3 |
| Density of Solid: | 8900 kg·m3 |
| Specific Heat: | 0.109 Cal/g/K @ 25°C |
| Superconductivity Temperature: | N/A |
| Triple Point: | N/A |
| Critical Point: | N/A |
| Heat of Fusion (kJ·mol-1): | 15.2 |
| Heat of Vaporization (kJ·mol-1): | 382.4 |
| Heat of Atomization (kJ·mol-1): | 423.082 |
| Thermal Conductivity: | 1.0 W/cm/K @ 298.2 K |
| Thermal Expansion: | (25 °C) 13.0 µm·m-1·K-1 |
| Electrical Resistivity: | 6.24 µΩ·m @ 20°C |
| Tensile Strength: | N/A |
| Molar Heat Capacity: | 24.81 J·mol-1·K-1 |
| Young's Modulus: | 209 GPa |
| Shear Modulus: | 75 GPa |
| Bulk Modulus: | 180 GPa |
| Poisson Ratio: | 0.31 |
| Mohs Hardness: | 5 |
| Vickers Hardness: | 1043 MPa |
| Brinell Hardness: | 700 MPa |
| Speed of Sound: | (20 °C) 4720 m·s-1 |
| Pauling Electronegativity: | 1.88 |
| Sanderson Electronegativity: | 2.56 |
| Allred Rochow Electronegativity: | 1.7 |
| Mulliken-Jaffe Electronegativity: | N/A |
| Allen Electronegativity: | N/A |
| Pauling Electropositivity: | 2.12 |
| Reflectivity (%): | 67 |
| Refractive Index: | N/A |
| Electrons: | 27 |
|---|---|
| Protons: | 27 |
| Neutrons: | 32 |
| Electron Configuration: | [Ar] 3d7 4s2 |
| Atomic Radius: | 125 pm |
| Atomic Radius,non-bonded (Å): | 2 |
| Covalent Radius: | 126±3 (low spin), 150±7 (high spin) pm |
| Covalent Radius (Å): | 1.18 |
| Van der Waals Radius: | 200 pm |
| Oxidation States: | 5, 4, 3, 2, 1, -1 (amphoteric oxide) |
| Phase: | Solid |
| Crystal Structure: | Hexagonal |
| Magnetic Ordering: | ferromagnetic |
| Electron Affinity (kJ·mol-1) | 63.851 |
| 1st Ionization Energy: | 760.41 kJ·mol-1 |
| 2nd Ionization Energy: | 1648.27 kJ·mol-1 |
| 3rd Ionization Energy: | 3232.28 kJ·mol-1 |
| CAS Number: | 7440-48-4 |
|---|---|
| EC Number: | 231-158-0 |
| MDL Number: | MFCD00010935 |
| Beilstein Number: | N/A |
| SMILES Identifier: | [Co] |
| InChI Identifier: | InChI=1S/Co |
| InChI Key: | GUTLYIVDDKVIGB-UHFFFAOYSA-N |
| PubChem CID: | 104730 |
| ChemSpider ID: | 94547 |
| Earth - Total: | 840 ppm |
|---|---|
| Mercury - Total: | 1690 ppm |
| Venus - Total: | 820 ppm |
| Earth - Seawater (Oceans), ppb by weight: | 0.08 |
| Earth - Seawater (Oceans), ppb by atoms: | 0.008 |
| Earth - Crust (Crustal Rocks), ppb by weight: | 30000 |
| Earth - Crust (Crustal Rocks), ppb by atoms: | 10000 |
| Sun - Total, ppb by weight: | 4000 |
| Sun - Total, ppb by atoms: | 70 |
| Stream, ppb by weight: | 0.2 |
| Stream, ppb by atoms: | 0.003 |
| Meterorite (Carbonaceous), ppb by weight: | 600000 |
| Meterorite (Carbonaceous), ppb by atoms: | 200000 |
| Typical Human Body, ppb by weight: | 20 |
| Typical Human Body, ppb by atom: | 2 |
| Universe, ppb by weight: | 3000 |
| Universe, ppb by atom: | 60 |
| Discovered By: | Georg Brandt |
|---|---|
| Discovery Date: | 1732 |
| First Isolation: | N/A |
Health, Safety & Transportation Information for Cobalt
Review and Print SDS for Cobalt Metal
SECTION 1. IDENTIFICATION
Product Name: Cobalt Metal
Product Number: All applicable American Elements product codes, e.g. CO-M-02, CO-M-03, CO-M-04, CO-M-05
CAS #: 7440-48-4
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
1093 Broxton Ave. Suite 2000
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America +1 800-424-9300
International +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
GHS08 Health hazard
Resp. Sens. 1 H334 May cause allergy or asthma symptoms or breathing difficulties if inhaled.
GHS07
Skin Sens. 1 H317 May cause an allergic skin reaction.
Classification according to Directive 67/548/EEC or Directive 1999/45/EC
Xn; Sensitizing
R42/43: May cause sensitization by inhalation and skin contact.
R53: May cause long-term adverse effects in the aquatic environment.
Information concerning particular hazards for human and environment:
Not applicable
Hazards not otherwise classified
No information known.
Label elements
Labelling according to Regulation (EC) No 1272/2008
The substance is classified and labeled according to the CLP regulation.
Hazard pictograms

GHS08
Signal word
Danger
Hazard statements
H334 May cause allergy or asthma symptoms or breathing difficulties if inhaled.
H317 May cause an allergic skin reaction.
Precautionary statements
P284 In case of inadequate ventilation wear respiratory protection.
P261 Avoid breathing dust/fume/gas/mist/vapours/spray.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
P342+P311 If experiencing respiratory symptoms: Call a POISON CENTER/doctor/...
P363 Wash contaminated clothing before reuse.
P501 Dispose of contents/container in accordance with local/regional/
national/international regulations.
WHMIS classification
D2A - Very toxic material causing other toxic effects
Classification system
HMIS ratings (scale 0-4)
(Hazardous Materials Identification System)
HEALTH
FIRE
REACTIVITY
0
0
0
Health (acute effects) = 0
Flammability = 0
Physical Hazard = 0
Other hazards
Results of PBT and vPvB assessment
PBT:
Not applicable.
vPvB:
Not applicable.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical characterization: Substances
CAS# Description:
7440-48-4 Cobalt
Identification number(s):
EC number:
231-158-0
SECTION 4. FIRST AID MEASURES
Description of first aid measures
General information
No special measures required.
After inhalation
Supply fresh air and to be sure call for a doctor.
Seek medical treatment in case of complaints.
After skin contact
Generally the product does not irritate the skin.
After eye contact
Rinse opened eye for several minutes under running water. If symptoms persist, consult a doctor.
After swallowing
If symptoms persist consult doctor.
Information for doctor
Most important symptoms and effects, both acute and delayed
No further relevant information available.
Indication of any immediate medical attention and special treatment needed
No further relevant information available.
SECTION 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing agents
Special powder for metal fires. Do not use water.
For safety reasons unsuitable extinguishing agents
Water
Special hazards arising from the substance or mixture
If this product is involved in a fire, the following can be released:
Metal oxide fume
Advice for firefighters
Protective equipment:
No special measures required
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Not required.
Environmental precautions:
Do not allow material to be released to the environment without proper governmental permits.
Do not allow product to reach sewage system or any water course.
Do not allow to penetrate the ground/soil.
Methods and material for containment and cleaning up:
Dispose of contaminated material as waste according to section 13.
Prevention of secondary hazards:
No special measures required.
Reference to other sections
See Section 7 for information on safe handling
See Section 8 for information on personal protection equipment.
See Section 13 for disposal information.
SECTION 7. HANDLING AND STORAGE
Handling
Precautions for safe handling
Keep container tightly sealed.
Store in cool, dry place in tightly closed containers.
Ensure good ventilation at the workplace.
Prevent formation of dust.
Information about protection against explosions and fires:
No special measures required.
Conditions for safe storage, including any incompatibilities
Storage
Requirements to be met by storerooms and receptacles:
No special requirements.
Information about storage in one common storage facility:
Do not store together with acids.
Further information about storage conditions:
Keep container tightly sealed.
Store in cool, dry conditions in well sealed containers.
Specific end use(s)
No further relevant information available.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Additional information about design of technical systems:
No further data; see section 7.
Control parameters
Components with limit values that require monitoring at the workplace:
7440-48-4 Cobalt (100.0%)
PEL (USA) Long-term value: 0.1* mg/m³
as Co; *for metal dust and fume
REL (USA) Long-term value: 0.05 mg/m³
as Co; metal dust & fume
TLV (USA) Long-term value: 0.02 mg/m³
as Co, BEI
EL (Canada) Long-term value: 0.02 mg/m³
IARC 2B
EV (Canada) Long-term value: 0.1 mg/m³
Ingredients with biological limit values:
7440-48-4 Cobalt (100.0%)
BEI (USA) 15 µg/L
Medium: urine
Time: end of shift at end of workweek
Parameter: Cobalt (background)
1 µg/L
Medium: blood
Time: end of shift at end of workweek
Parameter: Cobalt (background, semi-quantitative)
Additional information:
No data
Exposure controls
Personal protective equipment
General protective and hygienic measures
The usual precautionary measures for handling chemicals should be followed.
Maintain an ergonomically appropriate working environment.
Breathing equipment:
Not required.
Protection of hands:
Not required.
Penetration time of glove material (in minutes)
Not determined
Eye protection:
Safety glasses
Body protection:
Protective work clothing
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
General Information
Appearance:
Form: Solid in various forms
Color: Grey
Odor: Odorless
Odor threshold: Not determined.
pH-value: Not applicable.
Change in condition
Melting point/Melting range: 1495 °C (2723 °F)
Boiling point/Boiling range: 2927 °C (5301 °F)
Sublimation temperature / start: Not determined
Flammability (solid, gaseous)
Not determined.
Ignition temperature: Not determined
Decomposition temperature: Not determined
Auto igniting: Not determined.
Danger of explosion: Not determined.
Explosion limits:
Lower: Not determined
Upper: Not determined
Vapor pressure at 994 °C (1821 °F): 0.0000001 hPa
Density at 20 °C (68 °F): 8.92 g/cm³ (74.437 lbs/gal)
Bulk density at 20 °C (68 °F): 850 kg/m³
Relative density
Not determined.
Vapor density
Not applicable.
Evaporation rate
Not applicable.
Solubility in / Miscibility with Water: Insoluble
Partition coefficient (n-octanol/water): Not determined.
Viscosity:
dynamic: Not applicable.
kinematic: Not applicable.
Other information
No further relevant information available.
SECTION 10. STABILITY AND REACTIVITY
Reactivity
No information known.
Chemical stability
Stable under recommended storage conditions.
Thermal decomposition / conditions to be avoided:
Decomposition will not occur if used and stored according to specifications.
Possibility of hazardous reactions
No dangerous reactions known
Conditions to avoid
No further relevant information available.
Incompatible materials:
Acids
Hazardous decomposition products:
Metal oxide fume
SECTION 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity:
The Registry of Toxic Effects of Chemical Substances (RTECS) contains acute toxicity data for components in this product.
LD/LC50 values that are relevant for classification:
Oral LD50 6171 mg/kg (rat)
Skin irritation or corrosion:
No irritant effect.
Eye irritation or corrosion:
May cause irritation
Sensitization:
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
May cause an allergic skin reaction.
Germ cell mutagenicity:
The Registry of Toxic Effects of Chemical Substances (RTECS) contains mutation data for this substance.
Carcinogenicity:
IARC-2B: Possibly carcinogenic to humans: limited evidence in humans in the absence of
sufficient evidence in experimental animals.
ACGIH A3: Animal carcinogen: Agent is carcinogenic in experimental animals at a relatively high dose, by route(s) of administration, at site(s), of histologic type(s), or by mechanism(s) not considered relevant to worker exposure.
Available epidemologic studies do not confirm an increased risk of cancer in exposed humans.
Available evidence suggests that the agent is not likely to cause cancer in humans except under uncommon or unlikely routes or levels of exposure.
The Registry of Toxic Effects of Chemical Substances (RTECS) contains tumorigenic and/or carcinogenic and/or neoplastic data for this substance.
Reproductive toxicity:
No effects known.
Specific target organ system toxicity - repeated exposure:
No effects known.
Specific target organ system toxicity - single exposure:
No effects known.
Aspiration hazard:
No effects known.
Subacute to chronic toxicity:
No effects known.
Additional toxicological information:
To the best of our knowledge the acute and chronic toxicity of this substance is not fully known.
SECTION 12. ECOLOGICAL INFORMATION
Toxicity
Aquatic toxicity:
No further relevant information available.
Persistence and degradability
No further relevant information available.
Bioaccumulative potential
No further relevant information available.
Mobility in soil
No further relevant information available.
Additional ecological information:
General notes:
Do not allow material to be released to the environment without proper governmental permits.
Do not allow undiluted product or large quantities to reach ground water, water course or sewage system.
May cause long lasting harmful effects to aquatic life.
Avoid transfer into the environment.
Results of PBT and vPvB assessment
PBT:
Not applicable.
vPvB:
Not applicable.
Other adverse effects
No further relevant information available.
SECTION 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Recommendation
Consult state, local or national regulations to ensure proper disposal.
Uncleaned packagings:
Recommendation:
Disposal must be made according to official regulations.
SECTION 14. TRANSPORT INFORMATION
UN-Number
DOT, ADN, IMDG, IATA
Not applicable
UN proper shipping name
DOT, ADN, IMDG, IATA
Not applicable
Transport hazard class(es)
DOT, ADR, ADN, IMDG, IATA
Class
Not applicable
Packing group
DOT, IMDG, IATA
Not applicable
Environmental hazards:
Not applicable.
Special precautions for user
Not applicable.
Transport in bulk according to Annex II of MARPOL73/78 and the IBC Code
Not applicable.
Transport/Additional information:
DOT
Marine Pollutant (DOT):
No
SECTION 15. REGULATORY INFORMATION
Safety, health and environmental regulations/legislation specific for the substance or mixture
National regulations
All components of this product are listed in the U.S. Environmental Protection Agency Toxic Substances Control Act Chemical substance Inventory.
All components of this product are listed on the Canadian Domestic Substances List (DSL).
SARA Section 313 (specific toxic chemical listings)
7440-48-4 Cobalt
California Proposition 65
Prop 65 - Chemicals known to cause cancer
7440-48-4 Cobalt
Prop 65 - Developmental toxicity
Substance is not listed.
Prop 65 - Developmental toxicity, female
Substance is not listed.
Prop 65 - Developmental toxicity, male
Substance is not listed.
Information about limitation of use:
For use only by technically qualified individuals.
This product is subject to the reporting requirements of section 313 of the Emergency Planning and Community Right to Know Act of 1986 and 40CFR372.
Other regulations, limitations and prohibitive regulations
Substance of Very High Concern (SVHC) according to the REACH Regulations (EC) No. 1907/2006.
Substance is not listed.
The conditions of restrictions according to Article 67 and Annex XVII of the Regulation (EC) No 1907/2006 (REACH) for the manufacturing, placing on the market and use must be observed.
Substance is not listed.
Annex XIV of the REACH Regulations (requiring Authorisation for use)
Substance is not listed.
REACH - Pre-registered substances
Substance is listed.
Chemical safety assessment:
A Chemical Safety Assessment has not been carried out.
16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2016 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
Cobalt Isotopes
Naturally occurring cobalt has 1 stable isotope: 59Co.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 47Co | 47.01149(54)# | N/A | Unknown | 7/2-# | N/A | 339.16 | - |
| 48Co | 48.00176(43)# | N/A | p to 47Fe | (6+) | N/A | 356.55 | - |
| 49Co | 48.98972(28)# | <35 ns | p to 48Fe | 7/2-# | N/A | 375.81 | - |
| 50Co | 49.98154(18)# | 44(4) ms | ß+ + p to 49Mn; ß+ to 50Fe | (6+) | N/A | 391.34 | - |
| 51Co | 50.97072(16)# | 60# ms [>200 ns] | p to 51Fe | 7/2-# | N/A | 409.67 | - |
| 52Co | 51.96359(7)# | 115(23) ms | p to 52Fe | 0+ | N/A | 424.27 | - |
| 53Co | 52.954219(19) | 242(8) ms | p to 53Fe | 7/2- | N/A | 440.73 | - |
| 54Co | 53.9484596(8) | 193.28(7) ms | p to 54Fe | 4+ | N/A | 454.4 | - |
| 55Co | 54.9419990(8) | 17.53(3) h | EC to 55Fe | 7/2- | 4.822 | 469 | - |
| 56Co | 55.9398393(23) | 77.233(27) d | EC to 56Fe | 2+ | 3.85 | 478.94 | - |
| 57Co | 56.9362914(8) | 271.74(6) d | EC to 57Fe | 7/2- | 4.72 | 489.82 | - |
| 58Co | 57.9357528(13) | 70.86(6) d | EC to 58Fe | 5+ | 4.04 | 498.83 | - |
| 59Co | 58.9331950(7) | STABLE | - | 7/2- | 4.627 | 508.77 | 100 |
| 60Co | 59.9338171(7) | 5.2713(8) y | ß- to 60Ni | 2+ | 3.799 | 516.85 | - |
| 61Co | 60.9324758(10) | 1.650(5) h | ß- to 61Ni | 7/2- | N/A | 525.86 | - |
| 62Co | 61.934051(21) | 1.50(4) min | ß- to 62Ni | 1+ | N/A | 532.07 | - |
| 63Co | 62.933612(21) | 26.9(4) s | ß- to 63Ni | (7/2)- | N/A | 541.08 | - |
| 64Co | 63.935810(21) | 0.30(3) s | ß- to 64Ni | (3+) | N/A | 547.3 | - |
| 65Co | 64.936478(14) | 1.20(6) s | ß- to 65Ni | (7/2-)# | N/A | 554.44 | - |
| 66Co | 65.93976(27) | 0.18(1) s | ß- to 66Ni | (7-) | N/A | 559.73 | - |
| 67Co | 66.94089(34) | 0.425(20) s | ß- to 67Ni | 7/2-# | N/A | 566.88 | - |
| 68Co | 67.94487(34) | 0.199(21) s | ß- to 68Ni | (6-) | N/A | 571.23 | - |
| 69Co | 68.94632(36) | 227(13) ms | ß- to 69Ni; ß- + n to 68Ni | 7/2-# | N/A | 577.44 | - |
| 70Co | 69.9510(9) | 119(6) ms | ß- to 70Ni; ß- + n to 69Ni | (6-,7-) | N/A | 580.86 | - |
| 71Co | 70.9529(9) | 97(2) ms | ß- to 71Ni; ß- + n to 70Ni | 7/2-# | N/A | 588.01 | - |
| 72Co | 71.95781(64)# | 62(3) ms | ß- to 72Ni; ß- + n to 71Ni | 0+ | N/A | 591.43 | - |
| 73Co | 72.96024(75)# | 41(4) ms | Unknown | 7/2-# | N/A | 596.72 | - |
| 74Co | 73.96538(86)# | 50# ms [>300 ns] | Unknown | 0 | N/A | 600.14 | - |
| 75Co | 74.96833(86)# | 40# ms [>300 ns] | Unknown | 7/2 | N/A | 605.42 | - |