Osmium (original) (raw)
Data Zone | Discovery | Facts | Appearance & Characteristics | Uses | Abundance & Isotopes | References
The chemical element osmium is classed as a transition metal. It was discovered in 1803 by Smithson Tennant.

Data Zone
| Classification: | Osmium is a transition metal |
|---|---|
| Color: | bluish-white |
| Atomic weight: | 190.2 |
| State: | solid |
| Melting point: | 3030 oC, 3303 K |
| Boiling point: | 5012 oC, 5285 K |
| Electrons: | 76 |
| Protons: | 76 |
| Neutrons in most abundant isotope: | 116 |
| Electron shells: | 2,8,18,32,14,2 |
| Electron configuration: | [Xe] 4f14 5d6 6s2 |
| Density @ 20oC: | 22.61 g/cm3 |
Show more, including: Heats, Energies, Oxidation,
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 8.49 cm3/mol |
|---|---|
| Structure: | hcp: hexagonal close pkd |
| Hardness: | 7.0 mohs |
| Specific heat capacity | 0.13 J g-1 K-1 |
| Heat of fusion | 31.80 kJ mol-1 |
| Heat of atomization | 789 kJ mol-1 |
| Heat of vaporization | 627.6 kJ mol-1 |
| 1st ionization energy | 840 kJ mol-1 |
| 2nd ionization energy | 1600 kJ mol-1 |
| 3rd ionization energy | kJ mol-1 |
| Electron affinity | 104 kJ mol-1 |
| Minimum oxidation number | -2 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 8 |
| Max. common oxidation no. | 4 |
| Electronegativity (Pauling Scale) | 2.2 |
| Polarizability volume | 8.5 Å3 |
| Reaction with air | mild, ⇒ OsO4 |
| Reaction with 15 M HNO3 | mild, ⇒ OsO2 |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | OsO2, OsO4 |
| Hydride(s) | none |
| Chloride(s) | OsCl3, OsCl4, OsCl5 |
| Atomic radius | 135 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 87.6 W m-1 K-1 |
| Electrical conductivity | 12.3 x 106 S m-1 |
| Freezing/Melting point: | 3030 oC, 3303 K |
The Six Platinum Group Metals
Osmium is one of the platinum group metals. These metals have similar properties and are often present in the same mineral ores – osmium and iridium were found by Smithson Tennant in a sample of crude platinum.
Discovery of Osmium
Osmium was discovered in 1803, in London, by English chemist Smithson Tennant. He also discovered iridium in the same year. (3) Earlier in his career, Smithson Tennant had established that diamond is pure carbon.
Tennant’s discovery of osmium began when he dissolved a sample of crude platinum in aqua regia, a mixture of hydrochloric acid and nitric acid, resulting in a metallic, black powder.
Previous chemists had believed this powder was graphite, but Tennant – who had previous experience of working with carbon’s allotropes – thought differently. (3)
Tennant treated the powder with sodium hydroxide and heated to the solution. He removed an alkali from the residue by adding water. He added hydrochloric acid to the remaining residue to form an acidic solution. The alkaline solution was found to contain osmium, while the acidic contained iridium. (4)
The highly toxic osmium tetroxide, OsO4, has a characteristic unpleasant odor, so Tennant named the element after the Greek word for smell, ‘osme’.
In the image below, on the left is an electron microscopy image of osmium catalyst clusters containing nominally 5 atoms of osmium each, dispersed on a magnesium oxide support material. Image by ORNL (1). On the right, an osmium bead. Image by Tomihadnorf (2)


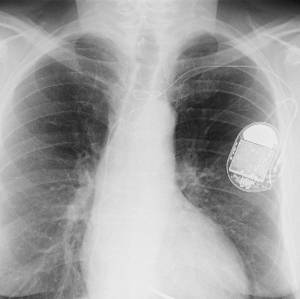
Alloys of platinum and osmium are used in the construction of pacemaker electrodes because they are highly resistant to corrosion. Photo by Sunzi99.
Appearance and Characteristics
Harmful effects:
Powdered osmium in air forms the pungent, highly toxic osmium tetroxide (OsO4) which can cause lung, skin and eye damage.
Characteristics:
Osmium is a rare, lustrous, very hard, brittle, bluish-white metal.
It is the densest of all the elements. (Although osmium’s density is very similar to iridium’s, osmium’s is slightly higher – both measured and calculated.
Calculated: Osmium 22.587 ± 0.009 g/cm3 & Iridium: 22.562 ± 0.009 g/cm3 at 20 OC. See data source.)
Osmium has the highest melting point and the lowest vapor pressure of the platinum group (ruthenium, rhodium, palladium, iridium, and platinum).
Uses of Osmium
Osmium is principally used alloyed with other metals in the platinum group to produce very hard alloys.
An alloy of 90% platinum and 10% osmium is used in surgical implants such as pacemakers and replacement heart valves.
Osmium tetroxide is used in microscopy as a stain for fatty tissue and in fingerprint detection.
Abundance and Isotopes
Abundance earth’s crust: 1.5 parts per billion by weight, 0.2 parts per billion by moles
Abundance solar system: 2 parts per billion by weight, 20 parts per trillion by moles
Cost, pure: $7700 per 100g
Cost, bulk: per 100g
Source: Osmium is found in platinum ores and in the mineral osmiridium (an alloy of osmium and iridium). Commercially, osmium is recovered as a by-product of nickel refining.
Isotopes: Osmium has 34 isotopes whose half-lives are known, with mass numbers from 162 to 196. Naturally occurring osmium is a mixture of seven isotopes and they are found in the percentages shown: 184Os (0.02%), 186Os (1.6%), 187Os (2.0%), 188Os (13.2%), 189Os (16.1%), 190Os (26.3%) and 192Os (40.8%). Naturally the most common isotope is 192Os, with an abundance of 40.8%.

References
- Photo: ORNL
- Photo: Tomihahndorf
- T. Tegg, First Lines of Science, 1827, James Mitchell, p205
- Jacob Green, Edward Turner, A Text Book of Chemical Philosophy, 1829, R.H. Small, p325
Cite this Page
For online linking, please copy and paste one of the following:
or
To cite this page in an academic document, please use the following MLA compliant citation:
"Osmium." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web.
https://www.chemicool.com/elements/osmium.html.