Sulfur (original) (raw)
Data Zone | Discovery | Facts | Appearance & Characteristics | Uses | Abundance & Isotopes | References
The chemical element sulfur is classed as a chalcogen and a nonmetal. It has been known since ancient times. Its discoverer and discovery date are unknown.

Data Zone
| Classification: | Sulfur is a chalcogen and a nonmetal |
|---|---|
| Color: | yellow |
| Atomic weight: | 32.06 |
| State: | solid |
| Melting point: | 115.2 oC, 388.4 K |
| Boiling point: | 444.7 oC, 717.9 K |
| Electrons: | 16 |
| Protons: | 16 |
| Neutrons in most abundant isotope: | 16 |
| Electron shells: | 2,8,6 |
| Electron configuration: | 1s2 2s2 2p6 3s2 3p4 |
| Density @ 20oC: | 2.07 g/cm3 |
Show more, including: Heats, Energies, Oxidation,
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 15.5 cm3/mol |
|---|---|
| Structure: | S8 rings |
| Hardness: | 2 mohs |
| Specific heat capacity | 0.71 J g-1 K-1 |
| Heat of fusion | 1.7175 kJ mol-1 |
| Heat of atomization | 279 kJ mol-1 |
| Heat of vaporization | 9.8 kJ mol-1 of S2 |
| 1st ionization energy | 999.6 kJ mol-1 |
| 2nd ionization energy | 2251 kJ mol-1 |
| 3rd ionization energy | 3360.6 kJ mol-1 |
| Electron affinity | 200.4144 kJ mol-1 |
| Minimum oxidation number | -2 |
| Min. common oxidation no. | -2 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 6 |
| Electronegativity (Pauling Scale) | 2.58 |
| Polarizability volume | 2.9 Å3 |
| Reaction with air | vigorous, w/ht ⇒ SO2 |
| Reaction with 15 M HNO3 | vigorous, ⇒ H2SO4, NOx |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | SO2, SO3 |
| Hydride(s) | H2S (hydrogen sulfide) |
| Chloride(s) | S2Cl2, SCl2 |
| Atomic radius | 100 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | 170 pm |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 0.205 W m-1 K-1 |
| Electrical conductivity | 5.0 x 10-14 S cm-1 |
| Freezing/Melting point: | 115.2 oC, 388.4 K |

Deposits of sulfur around a volcanic vent

Most health benefits from onions and garlic come from sulfur compounds.

Sulfur plains spreading around an eruption on Jupiter’s volcanic moon, Io. Photo: Nasa

Ancient sulfur refining: Sulfur ore is heated in pots whose lids fit tightly to limit the release of SO2. (The modern short-term exposure limit for SO2 is 5 ppm. (12) ) Liquid sulfur is collected below the pots. Georgius Agricola, 1556.
Discovery of Sulfur
Sulfur has been known since ancient times. In the Bible it is called brimstone. It can be found in its elemental state around volcano vents.
The name may have been derived from the Arabic ‘sufra’ meaning yellow, or the Sanskrit ‘shulbari’ meaning enemy (ari) of copper (shulba). (1)
The Sanskrit possibility is appealing, because it carries a message about people’s knowledge of chemistry from long ago: sulfur actually does react easily with many metals, including copper. (Sanskrit is one of the oldest Indo-European languages – over 3000 years old. Despite this, it is the human language most compatible with artificial intelligence. (2))
When sulfur burns it produces sulfur dioxide, a poisonous gas. At one time this gas was used in New York to fumigate buildings harboring infectious diseases. (3)
The use of burning sulfur for fumigation began several thousand years ago. In Homer’s ‘The Odyssey’ which is about 2800 years old, Odysseus says, “Bring sulfur, old nurse, that cleanses all pollution, and bring me fire, that I may purify the house with sulfur…” (4)
In the year 808 a Chinese text provides us with possibly the first recipe for gunpowder, containing saltpeter, sulfur and carbon. (5)
Sulfur is also believed to have been a component of ‘Greek Fire,’ a weapon similar to a flamethrower used by the Byzantine Empire. (6), (7)
Sulfur became a recognized chemical element in 1789, when Antoine Lavoisier included it in his famous list of the elements. (8)
In 1823, German chemist Eilhard Mitscherlich discovered sulfur’s allotropy: he showed that the crystal shapes of sulfur obtained from cooling molten sulfur were different from those obtained when the element crystallized from a solution. (9)
The sulfur obtained from molten sulfur is called monoclinic sulfur, while sulfur obtained from crystallizing a solution is called rhombic sulfur. Both forms consist of S8 rings. The difference between the forms is the way the rings are arranged within a crystal.
At this time the concept of allotropy – different structural forms of the same element – had not become a formal part of chemistry. It was not until 1841 that Berzelius introduced the term to explain sulfur’s monoclinic and rhombic forms. (10)
By the 1800s sulfur, in the form of sulfuric acid, had become the best way to judge a country’s wealth. Countries had even gone to war over sulfur.
Here’s what the great German chemist Justus Liebig had to say about it in about 1843:
“It is no exaggeration to say, we may fairly judge the commercial prosperity of a country from the amount of sulfuric acid it consumes.
(Sulfur’s price affects the price of…) bleached and printed cotton stuffs, soap, glass, etc, and remembering that Great Britain supplies America, Spain, Portugal, and the East, with these, exchanging them for raw cotton, silk, wine, raisins, indigo, etc, we can understand why the English Government should have resolved to resort to war with Naples (in 1839) in order to abolish the sulfur monopoly, which the latter power attempted recently to establish.” (11)
Interesting Facts about Sulfur
- Sulfur makes up almost 3% of the earth’s mass. If you think that’s not much, next time you look to the sky and see the moon, think of this: the earth contains enough sulfur to make not just one new moon, but two!
- When Shakespeare’s Othello asks for punishment, one possibility he mentions is: “…roast me in sulphur!”
- Sulfur burns with a very satisfying blue flame – its old name is brimstone, which means ‘burn stone’ or ‘stone that burns.’
- Pure sulfur has no smell, but many of its compounds stink! For example sulfur compounds called mercaptans give skunks their awful smell. Rotten eggs (and most stink bombs) get their distinctive aroma courtesy of hydrogen sulfide, H2S.
- Certain cave bacteria digest hydrogen sulfide and produce snottites (think of slimy stalactites) in caves. These snottites drip sulfuric acid with a pH as low as zero – that’s enough to burn holes in your clothes if you stand underneath them. Snottite bacteria thrive in areas where there are sulfur deposits or sulfur-containing minerals or hydrocarbons. The sulfuric acid they excrete carves out new cave systems underground by dissolving rocks.
- There’s a much higher proportion of sulfur in the earth’s core than in its crust – approximately 100 times more.
- Penicillin is a natural, sulfur-based antibiotic.

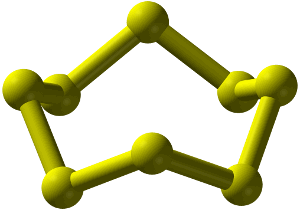
A sulfur 8 ring.
Sulfur reacts very vigorously with zinc. Here’s a test firing of a sulfur/zinc rocket.
Crystallization of liquid sulfur.
Appearance and Characteristics
Harmful effects:
Elemental sulfur is considered to be of low toxicity.
Compounds such as carbon disulfide, hydrogen sulfide, and sulfur dioxide are toxic. For example, at 0.03 parts per million, we can smell hydrogen sulfide but it is regarded as safe for eight hours of exposure. At 4 ppm it may cause eye irritation. At 20 ppm exposure for more than a minute causes severe injury to eye nerves. At 700 ppm breathing stops. Death will result if there is not a quick rescue. Permanent brain damage may result. (14)
Characteristics:
Sulfur is a soft, pale yellow, odorless, brittle solid. It is insoluble in water, but soluble in carbon disulfide. It burns with a blue flame, oxidizing to sulfur dioxide.
Sulfur exists in several crystalline and amorphous allotropes. The most common form is yellow, orthorhombic alpha-sulfur, which contains puckered rings of S8.
Sulfur is multivalent and combines, with valence 2, 4, or 6, with almost all other elements. The best known sulfur compound is hydrogen sulfide (H2S). This is a toxic gas that smells like rotten eggs; the smell is used in stink bombs, many of which release a small amount of hydrogen sulfide.
Uses of Sulfur
Sulfur’s main commercial use is as a reactant in the production of sulfuric acid (H2SO4). Sulfuric acid is the industrialized world’s number one bulk chemical, required in large quantities in lead-acid batteries for automotive use.
Sulfur is also used in the vulcanization of natural rubber, as a fungicide, in black gunpowder, in detergents and in the manufacture of phosphate fertilizers.
Sulfur is a vital element for all forms of life. It is a component of two amino acids, cysteine and methionine.
Abundance and Isotopes
Abundance earth’s crust: 350 parts per million by weight, 225 parts per million by moles
Abundance solar system: 400 parts per million by weight, 10 parts per million by moles
Cost, pure: $50 per 100g
Cost, bulk: $ per 100g
Source: Sulfur deposits are found naturally in areas around hot springs and in volcanic regions. It is also widely found in nature as iron pyrites (iron sulfide), galena (lead sulfide), gypsum (calcium sulfate), Epsom salts (magnesium sulfate) and many other minerals.
Sulfur is recovered commercially from underground deposits using the Frasch Process – superheated water and steam are pumped underground, where they melt the sulfur, allowing it to be pumped to the surface. Sulfur is also obtained commercially as a by-product of refining crude oil.
Isotopes: Sulfur has 18 isotopes whose half-lives are known, with mass numbers 27 to 45. Naturally occurring sulphur is a mixture of its four stable isotopes and they are found in the percentages shown: 32S (95.0%), 33S (0.8%), 34S (4.2%), and 36S (0.02%).

References
- G. Eggert, M. Weichert, H. Euler, B. Barbier, Some news about Black Spots., 2004, Proceedings of Metal, p142 (pdf download).
- Rick Briggs, Knowledge Representation in Sanskrit and Artificial Intelligence., AI Magazine Volume 6 Number 1, 1985, p32.
- Cyrus Edson, Disinfection of Dwellings by Means of Sulphur Dioxide., Public Health Pap Rep., 1889, 15: p65-68.
- Thomas F. Glick, Steven John Livesey, Faith Wallis, Dioxide, Medieval Science, Technology, and Medicine: An Encyclopedia., 2005, p211, Routelidge.
- Homer, The Odyssey, p270.
- Charles Stephenson, The Admiral’s Secret Weapon: Lord Dundonald and the Origins of Chemical Warfare., p93, Boydell Press.
- Eric Croddy, Chemical and Biological Warfare., p128, Copernicus Books
- Antoine Lavoisier, Elements of Chemistry at Project Gutenberg 1790, Translation of the original 1789 French by Robert Kerr.
- Hans-Werner Schütt, Eilhard Mitscherlich, Prince of Prussian Chemistry., p98, Chemical Heritage Foundation.
- William B. Jensen, The Origin of the Term Allotrope., J. Chem. Educ., 2006, 83 (6), p838.
- Justus Freiherr von Liebig, Familiar Letters on Chemistry., 1843.
- Sulfur Dioxide in Workplace Atmospheres Occupational Safety & Health Administration
- Why does chopping an onion make you cry?, Library of Congress.
- Toxicity of hydrogen sulfide gas.
Cite this Page
For online linking, please copy and paste one of the following:
or
To cite this page in an academic document, please use the following MLA compliant citation:
"Sulfur." Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web.
https://www.chemicool.com/elements/sulfur.html.