Atomic Weight of Tin | Commission on Isotopic Abundances and Atomic Weights (original) (raw)
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
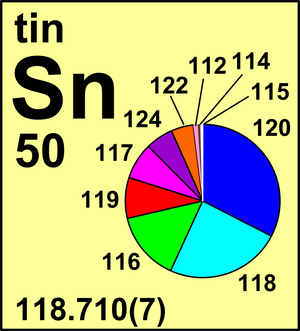
| 112Sn | 111.904 825(2) | 0.0097(1) |
| 114Sn | 113.902 7801(2) | 0.0066(1) |
| 115Sn | 114.903 3447(1) | 0.0034(1) |
| 116Sn | 115.901 7428(6) | 0.1454(9) |
| 117Sn | 116.902 954(3) | 0.0768(7) |
| 118Sn | 117.901 607(3) | 0.2422(9) |
| 119Sn | 118.903 311(5) | 0.0859(4) |
| 120Sn | 119.902 202(6) | 0.3258(9) |
| 122Sn | 121.903 44(2) | 0.0463(3) |
| 124Sn | 123.905 277(7) | 0.0579(5) |
In its 1961 report, the Commission recommended _A_r(Sn) = 118.69 based on chemical-ratio determinations. From these measurements, with current values of the atomic weights of the other elements involved, the following atomic weights for Sn are derived: _A_r(Sn) = 118.686, 118.691, and 118.701. The Commission was also aware that three mass-spectrometric determinations had been made that yield slightly higher atomic-weight values.
Tin has ten stable isotopes, the largest number of all elements. Because of this, the isotopic composition measurements involve an unusually large number of experimentally determined ratios, each subject to uncertainty. In 1969, the Commission assessed _A_r(Sn) = 118.69(3) therefore preferring the chemically determined atomic-weight values. This viewpoint was reaffirmed until 1983 when the Commission was able to consider the first calibrated mass-spectrometric measurement, which reported _A_r(Sn) = 118.7099(22) and demonstrated good agreement with many previous isotope-abundance measurements (after correcting those uncalibrated measurements for isotope fractionation). In 1983 the Commission changed the basis for the standard atomic weight of tin to mass spectrometry and the value to _A_r(Sn) = 118.710(7).
The "g" annotation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, southwest Africa.
SOURCE Atomic weights of the elements: Review 2000 by John R de Laeter et al. Pure Appl. Chem. 2003 (75) 683-800
© IUPAC 2003
CIAAW
Tin
_A_r(Sn) = 118.710(7) since 1983
The name derives from the Anglo-Saxon tin of unknown origin. The symbol Sn is derived from Latin stannum for alloys containing lead. The element was known in prehistoric times.