Updated advances of linking psychosocial factors and sex hormones with systemic lupus erythematosus susceptibility and development (original) (raw)
Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that primarily affects women, especially those of reproductive age. Genetics, environment, and gene-environment interactions play key roles in the development of SLE. Despite the numerous susceptibility genes of SLE identified to date, gene therapy is far from a clinical reality. Thus, more attention should be paid to the risk factors and underlying mechanisms of SLE. Currently, it is reported that psychosocial factors and sex hormones play vital roles in patients with SLE, which still need further investigated. The purpose of this review is to update the roles and mechanisms of psychosocial factors and sex hormones in the susceptibility and development of SLE. Based on review articles and reports in reputable peer-reviewed journals and government websites, this paper summarized psychosocial factors (e.g., alexithymia, depression, anxiety, negative emotions, and perceived stress) and sex hormones (e.g., estrogens, progesterone, androgens, and prolactin) involved in SLE. We further explore the mechanisms linking these factors with SLE susceptibility and development, which can guide the establishment of practical measures to benefit SLE patients and offer new ideas for therapeutic strategies.
Keywords: Systemic lupus erythematosus, Psychosocial factors, Sex hormones, Risk factors, Therapy targets
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with high heterogeneity that predominantly affects women, especially those of reproductive age (Mok, 2018). Several factors are linked to the pathogenesis of SLE, including genetic and environmental factors, along with gene-environment interactions (Mok, 2017). Although many SLE susceptibility genes have been identified through association studies, gene therapy is a long way from clinical application (Mok, 2017). The conventional therapy for SLE is based on a high dose of immunosuppressive drugs; however, the significant side effects associated with this treatment are causing increasing concern, calling for the urgent need to discover new treatment targets. Toward this end, it is essential to comprehensively consider the diversity of risk factors of SLE, and to investigate the underlying mechanisms contributing to these risks.
Currently, numerous studies report that psychosocial factors and sex hormones are important to SLE. However, the interactions between psychosocial factors and sex hormones and SLE are complicated, which still need further investigated. Therefore, this review is aimed to investigate the roles and effects of psychosocial factors and sex hormones in SLE. To facilitate this field of study, we here provide a systematic review of the effects of psychosocial factors focusing on alexithymia, depression, anxiety, negative emotions, and perceived stress, and sex hormones, focusing on estrogens, progesterone, androgens, and prolactin (PRL), in SLE, and explore the mechanisms contributing to these associations.
Survey methodology
This paper was based on review articles and reports in reputable peer-reviewed journals and government websites. The research was conducted using Medline on OvidSP, PubMed, Google Scholar, website, books, e-books, and reports. The words “systemic lupus erythematosus”, “psychosocial factors”, “sex hormones”, “alexithymia”, “depression”, “anxiety”, “negative emotions”, “perceived stress”, “estrogens”, “progesterone”, “androgens”, “prolactin” and a combination of those were used to retrieve literature from the databases.
Psychosocial factors in SLE
Disease activity can be triggered in patients with inactive SLE following experiences leading to negative emotions such as severe mental stimulation and excessive emotional repression. Moreover, substantial research on psychological factors of disease conducted in the last two decades has demonstrated a high frequency of alexithymia, characterized by an inability to recognize or describe personal emotions, among patients with chronic autoimmune diseases such SLE and rheumatoid arthritis (RA); this link was further associated with psychological distress and quality of life (QoL) impairment, thereby increasing the risk of medical or psychiatric diseases (Barbosa et al., 2009, 2011; Vadacca et al., 2008, 2014). In SLE, the link with alexithymia was found to be further related to adult attachment disorders (Barbasio & Granieri, 2013). The inability to develop negative emotions through emotional regulation contributes to direct somatic disturbances, which may enhance the physical symptoms in patients with SLE.
In recent years, depression and anxiety have also been suggested to play an important role in the activation of SLE, owing to their associations with proteinuria (Bai et al., 2016). In addition, other forms of depression have been suggested to occur in SLE patients, including minimal or mild depressive symptoms (Bai et al., 2016; Zhang et al., 2017). The form of the symptoms has been correlated with a complicated mix of biological, social, and psychological factors in SLE patients, with more severe depressive symptoms leading to a high average SLE disease activity index score (Alsowaida et al., 2018).
One hypothesis that has been put forward to explain these associations involves the influence of immune-neuro-endocrine factors in the pathogenesis of SLE and RA (Vadacca et al., 2008). Negative emotions have been suggested to influence the immune system via impacting the sympathetic nervous system and endocrine functions, leading to immune dysregulation (Barak, 2006; Dowlati et al., 2010; Matsunaga et al., 2008; Schiepers, Wichers & Maes, 2005; Solomon, Amkraut & Kasper, 1974). Indeed, neuropeptides and endocrine hormones play a crucial role in the pathogenesis of SLE. A multitude of studies revealed that an increased level of neuropeptides decreases the hypothalamic-pituitary-adrenal axis tone, giving rise to abnormalities of immune function and inflammatory processes, thereby contributing to the development of SLE both in patients and animal models (Bracci-Laudiero et al., 1998, 1999; Harle et al., 2006). Depression and perceived stress, as negative emotions, are mediated by feelings of helplessness originating from this altered immune function (Mills et al., 2018; Roberts et al., 2018). Moreover, some studies found that SLE patients frequently produced serum antibodies against N-methyl-D-aspartic acid receptors (Diamond et al., 2006), leading to structural and functional damage of the amygdala in patients and animal models of SLE, which in turn contribute to an associated deficit in emotional processing such as cognitive and emotional abnormalities (Diamond et al., 2006; Watson et al., 2012).
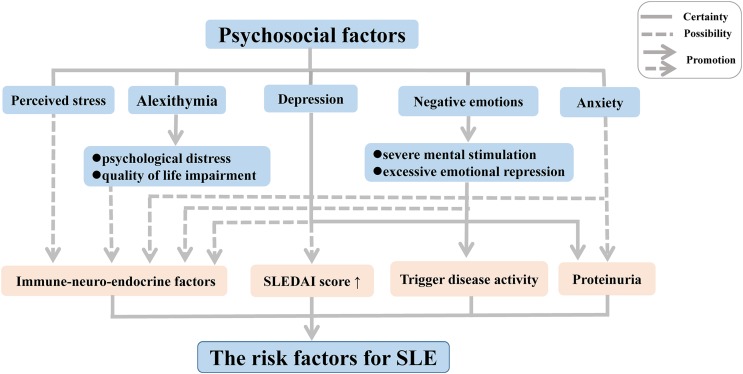
Although it has now become clear that psychosocial factors may influence the disease onset and progression of SLE independently and interactively (Fig. 1), the precise biological mechanisms that mediate these responses implicated in the development of SLE remain poorly understood. Thus, further research should focus on elucidation of the immune mechanisms related to alterations in mood and emotion in SLE, which will be indispensable to gain a comprehensive understanding of the complex pathogenesis.
Figure 1. Psychosocial factors as risk factors for SLE.
Abbreviation: SLEDAI score, SLE Disease Activity Index score.
Additionally, finding an efficient way to regulate the psychosocial factors may be good for the physical treatment of the patients with SLE. Mindfulness-based cognitive therapy (MBCT) is a treatment combining mindfulness meditation and interventions from cognitive therapy and is delivered in a group setting (Barnhofer et al., 2009). Recently, a clinical trial found that MBCT contributed to decreased psychological symptoms, such as depression, anxiety and alexithymia, and improved QoL, as well as benefited physical treatments in patients with SLE (Solati et al., 2017). Therefore, MBTC may be a promising intervention for treating psychosocial problems in clinical populations, including patients with SLE, although its efficacy is little known now. At the same time, there will be an increasing intervention to remove psychosocial symptoms for the patients with SLE.
Sex hormones in SLE
Most human autoimmune diseases show an increased incidence and prevalence in females; this is particularly true for SLE, which is exacerbated during puberty, pregnancy, and post-partum periods (Tedeschi, Bermas & Costenbader, 2013). Many sex hormones that are also known to affect the immune system (Parks et al., 2017; Pennell, Galligan & Fish, 2012), may play a role as triggers or protectors of the development of SLE (Sakiani, Olsen & Kovacs, 2013; Tedeschi, Bermas & Costenbader, 2013). Studies in human populations and experimental models have indicated an elevated risk of SLE associated with exposure to estrogen (Cunningham et al., 2014; Cutolo, Sulli & Straub, 2012; Hughes & Choubey, 2014; Sakiani, Olsen & Kovacs, 2013; Strickland et al., 2012), whereas progesterone and testosterone appear to play a protective role via counteracting the effects of estrogen (Hughes, 2012; Hughes & Choubey, 2014; Sakiani, Olsen & Kovacs, 2013; Tan, Peeva & Zandman-Goddard, 2015; Tsur et al., 2015). Although the risk of developing SLE from exposure to other sex hormones such as PRL remains controversial, a pathogenic role of PRL in SLE can be inferred based on epidemiological and experimental animal studies (Costanza et al., 2015; Karimifar et al., 2013; Orbach et al., 2012; Saha et al., 2011; Shelly, Boaz & Orbach, 2012). In addition, limited data suggest a role of luteinizing hormone releasing hormone, also known as gonadotropin releasing hormone, in the pathogenic mechanisms of SLE (Jacobson, 2000, 2001; Jacobson et al., 1999; Jacobson, Nisula & Steinberg, 1994; Walker & Jacobson, 2000). The influence of these sex hormones on SLE and their effects are discussed in detail below.
Estrogens
The potential role of estrogen in autoimmune diseases has been extensively investigated, particularly for SLE. The mode of action of estrogen and its metabolites differs with regards to the specific effects on the modulation of immunity (Cutolo, Sulli & Straub, 2012). It is well established that low doses of estrogen enhance T helper (Th)1 cell responses, whereas high estrogen levels raise Th2 responses (Beagley & Gockel, 2003; Salem, 2004). By contrast to estradiol (E2), estriol (E3) strongly provokes antibody production in response to bacteria (Ding & Zhu, 2008). In genetically predisposed individuals, estrogens increase the risk of disease and have a pathogenic role in SLE. The pathogenic mechanisms may involve the production of type 1 interferon (IFN), survival of auto-reactive B cells and production of pathogenic immunoglobulin (IgG) auto-antibodies, and/or differentiation of CD4+ Th cells (Sakiani, Olsen & Kovacs, 2013; Tabor & Gould, 2017). Estrogen signals act on the nuclear receptors estrogen receptor (ER)-α and ER-β in many types of immune cells (Cunningham & Gilkeson, 2011; Hill et al., 2011; Kovats, 2012; Moulton, 2018; Yakimchuk, Jondal & Okret, 2013). Enhanced expression of type 1 IFN-inducible genes has been detected in SLE, and this so-called “IFN signature” has been shown to play a critical role in the pathogenesis of SLE and is associated with active disease (Baccala et al., 2007; Baechler et al., 2003; Li et al., 2010; Ronnblom & Eloranta, 2013). Thus, active immunization with IFN-kinoid can downregulate this IFN signature in SLE (Lauwerys et al., 2013). In a mouse model, estrogens were suggested to increase the production level of IFN-α through up-regulation of genes regulating the production of type 1 IFN such as Ifi202 (Choubey, 2012; Choubey et al., 2011; Panchanathan et al., 2009), Unc93b1 (Panchanathan, Liu & Choubey, 2013), or Irf5 (Choubey et al., 2011; Shen et al., 2010). Simultaneously, type 1 IFNs also increase estrogen signaling in immune cells by provoking the expression of ER-α, thereby contributing to SLE development and progression (Choubey et al., 2011; Panchanathan et al., 2010).
Moreover, numerous studies have suggested that estrogens have various effects on B cell development, survival, and differentiation, and in regulating the production of pathogenic auto-antibodies (Hill et al., 2011). Estrogen promotes the survival of self-reactive B cells by preventing their elimination or inactivation at developmental checkpoints. This is possibly related to the up-regulated expression of Bcl-2, CD22, SRC homology region 2 domain-containing phosphatase-1 (SHP-1), vascular cell adhesion molecule 1 (VCAM-1), protein tyrosine phosphatase, non-receptor type 6 (PTPN6), and B-cell activating factor (BAFF) (Bassi et al., 2015; Bynoe, Grimaldi & Diamond, 2000; Grimaldi et al., 2002; Hill et al., 2011; Panchanathan & Choubey, 2013). However, selective estrogen receptor modulators raloxifene suppressed estrogen-mediated effects on the survival, differentiation, and activation of autoreactive B cells in NZB/WF1 mice, which might serve to ameliorate lupus activity (Zhang et al., 2010).
An Ig class switch occurs in B cells from IgM to IgG, which requires activation-induced cytidine deaminase (AICDA), and estrogens have been shown to increase the expression of AICDA and homeobox protein Hox-C4 (HOXC4) in B cells, leading to the development of pathogenic IgG auto-antibodies (Mai et al., 2010; Pauklin et al., 2009; Sakiani, Olsen & Kovacs, 2013). Estrogens can also enhance the production of anti-double-stranded DNA (dsDNA) antibody and IgG or IgM by peripheral blood mononuclear cells and serum, which enhances the disease severity causing flare-ups (Khan & Ansar Ahmed, 2016). Moreover, estrogen has multiple effects on the development, differentiation, and functions of CD4+ T cells (Walters et al., 2009). In SLE, estrogen induces activation of the T lymphocytes via ER-α and ER-β, and increases the expression of T cell activation markers such as CD154 and calcineurin (Lin et al., 2011; Rider et al., 2006). Estrogen was also shown to exacerbate lupus disease severity via an ERα-independent mechanism along with other immune effects contributing to lupus pathogenesis, including modulation of Toll-like receptor (TLR) pathways, dendritic cell development, or E2-TWEAK signaling (Scott et al., 2017). These mechanisms might also contribute to the pathogenic mechanism of SLE, primarily affecting women.
Progesterone
The relationship between progestogens and SLE is complex. Progesterone has been suggested to play an important protective role against SLE disease activity (Hughes, 2012; Hughes & Choubey, 2014; Tan, Peeva & Zandman-Goddard, 2015; Tsur et al., 2015). Progesterone can also counteract the effects of estrogens summarized above, such as the estrogen-induced increase in type 1 IFN production, survival of auto-reactive B cells and pathogenic IgG auto-antibodies production, and differentiation of CD4+ Th cells. Progesterone has immune-modulatory effects that generally have anti-inflammatory outcomes (Tait, Butts & Sternberg, 2008). In SLE, the promotion of IFN-α/β production is primarily controlled by IFN regulatory factors (IRFs) such as IRF-3, IRF-5, and IRF-7 (Baccala et al., 2007; Fu et al., 2011; Niewold et al., 2012; Ronnblom, Eloranta & Alm, 2006; Ronnblom & Pascual, 2008; Sigurdsson et al., 2005; Stone et al., 2012; Wang et al., 2013). Progestogens impair IRF-7 activation in plasmacytoid dendritic cells (Hughes et al., 2008), which regulate the TLR-mediated decreased production of IFN-α, a major source of type 1 IFN, through depot medroxyprogesterone acetate (DMPA), thereby ameliorating the IFN signature and consequently SLE disease activity (Tan, Peeva & Zandman-Goddard, 2015). Moreover, progesterone has the opposite effect of estrogen with regards to the Ig class switch of B cells, resulting in a decrease of the IgM to IgG class switch recombination via suppressing the transcription of AICDA (Park, 2012; Pauklin & Petersen-Mahrt, 2009; Pauklin et al., 2009; Sakiani, Olsen & Kovacs, 2013). Specifically, progesterone reduces the production of IFN-γ, which induces a class switch to IgG2a in B cells, leading to pathogenic IgG auto-antibodies production (Hughes, Clark & Wong, 2013). In the last decade, there has been much research focused on the promotion effect of progesterone for Th2 differentiation; in particular, high levels of progesterone will induce a Th2-type immunologic response and suppress a Th1-type response via inhibiting IL-12 signaling in SLE (Hughes, Clark & Wong, 2013; Miyaura & Iwata, 2002). Meanwhile, there is a decrease in mortality, glomerulonephritis and Th1-related autoantibody production after DMPA treatment in NZB/NZW mice, which suggest that progesterone may have therapeutic benefit for SLE patients (Hughes et al., 2009; Recalde et al., 2018).
Androgens
Androgens may play an important protective role in the pathogenic mechanisms contributing to the development of SLE. Similar to progesterone, androgens target key immune pathways that can protect against SLE by counteracting the effects of estrogens. In particular, androgens down-regulate the levels of Ifi202, which in turn decreases the production of type 1 IFN (Choubey, 2012; Choubey et al., 2011; Panchanathan et al., 2009). Compared with estrogens, androgens may enhance the checkpoints for the auto-reactivity of B cells to induce B cell apoptosis via decreasing the level of Bcl-2 in B cells (Altuwaijri et al., 2009), and can also increase the level of transforming growth factor beta 1 (TGF-β1) in marrow stromal cells (Olsen, Gu & Kovacs, 2001), thereby suppressing the development of autoimmunity (Gubbels Bupp & Jorgensen, 2018; Sakiani, Olsen & Kovacs, 2013; Zhu et al., 2016). Androgens have also been shown to decrease the level of pathogenic IgG auto-antibodies production through inhibition of Ig class switching (Sakiani, Olsen & Kovacs, 2013). Androgens decrease the Th1 differentiation of CD4+ T cells via inhibiting IL-12 signaling, which in turn mitigates CD4+ responses in autoimmune disease (Kissick et al., 2014). Androgens also appear to modulate the accumulation of Gr1+CD11b+ cells in male mice and could inhibit the function of T follicular helper cells, formation of the germinal center, and the differentiation of plasma cells (Bird et al., 2017). CD11b+ cells were shown to overexpress DHT-regulated genes and colony-stimulating factor 3 receptor (Gubbels Bupp, Jorgensen & Kotzin, 2008), which was identified to influence the development of lupus and RA (Elera-Fitzcarrald et al., 2017; Gubbels Bupp, Jorgensen & Kotzin, 2008).
Prolactin
Only very limited data are currently available on the contributions of PRL to the development of autoimmune diseases. Indeed, the relationship between PRL and SLE and the underlying mechanisms remain controversial; however, the general perception is that PRL does play a pathogenic role in SLE, particularly with respect to hyper-prolactinemia (Costanza et al., 2015; Karimifar et al., 2013; Shelly, Boaz & Orbach, 2012). Under a specific genetic background, like estrogen, PRL has also been shown to promote the survival of self-reactive B cells by impairing B cell receptor-mediated clonal deletion and decreasing B cell apoptosis, thus breaking down B cell self-tolerance, leading to the development of autoimmunity (Elera-Fitzcarrald et al., 2017; Gonzalez, Saha & Peeva, 2013; Orbach & Shoenfeld, 2007; Saha et al., 2009, 2011). In B6.Sle3 mice, PRL increases the expression of co-stimulatory molecules (CD40, CD86 (B7-2), and MHC II molecules) on B cells, leading to enhanced antibody responses (Gonzalez, Saha & Peeva, 2013; Matera, Mori & Galetto, 2001). With respect to the differentiation of CD4+ Th cells, PRL up-regulates the levels of INF-γ, IL-12, IL-2, and CD40L (Clevenger, Altmann & Prystowsky, 1991; Clevenger et al., 1990; Gonzalez, Saha & Peeva, 2013; Jara et al., 2017; Matalka, 2003; Mukherjee, Mastro & Hymer, 1990; Orbach et al., 2012), leading to CD4+ T cell activation that can drive the development of autoimmunity in SLE. Recent studies have also implicated PRL in STAT5 activation, and in the induction of Ig synthesis and anti-dsDNA antibodies in SLE. However, it is reported that bromocriptine, a drug that inhibits PRL secretion, abrogates some of the immune effects of PRL in mice (Saha et al., 2011). Actually, the association between PRL levels and disease activity in SLE is still controversial (Elera-Fitzcarrald et al., 2017). Thus, further research on these relationships is warranted.
Here, we updated the roles and the effects of sex hormones including estrogens, progesterone, androgens, PRL on SLE, and some feasible measures could suppress some of effects of sex hormones in SLE mice. Most importantly, the mechanisms linking these factors with SLE susceptibility and development were explored (Table 1), which can guide the establishment of practical measures to benefit SLE patients and offer new ideas for therapeutic strategies.
Table 1. Influence of sex hormones on SLE and their effects.
| Sex hormones | Regulation | Performance | Effects in SLE |
|---|---|---|---|
| Estrogens | _Ifi202_↑, _Unc93b1_↑, or _Irf5_↑ | IFN-α↑, type 1 IFN↑ | Pathogenic effect |
| Bcl-2↑, CD22↑, SHP-1↑, VCAM-1↑, PTPN6↑, BAFF↑ | Survival of auto-reactive B cells↑ | ||
| AICDA↑, HOXC4↑ | Pathogenic IgG auto-antibodies↑ | ||
| ER-α↑, ER-β↑, CD154↑, calcineurin↑ | Differentiation of CD4+ Th cells↑ | ||
| Progesterone | IRF-7 activation↑ | IFN-α↓, type 1 IFN↓ | Protective effects |
| IFN-γ↓, suppressing the transcription of AICDA | Pathogenic IgG auto-antibodies↓ | ||
| Inhibit IL-12 signaling | Induce Th2-type immunologic response and suppress Th1-type response | ||
| Androgens | _Ifi202_↓ | Type 1 IFN↓ | Protective effects |
| Bcl-2↓ | Survival of auto-reactive B cells↓, TGF-β1↑ | ||
| Inhibit Ig class switching | Pathogenic IgG auto-antibodies↓ | ||
| Inhibit IL-12 signaling | Th1 differentiation of CD4+ T cells↓ | ||
| Prolactin (PRL) | BCR-mediated clonal deletion, B cell apoptosis↓ | Survival of auto-reactive B cells↑ | Pathogenic effect |
| INF-γ↑, IL-12↑, IL-2↑, CD40L↑ | CD4+ T cell activation | ||
| Ig synthesis↑, anti-dsDNA antibodies↑ |
Conclusions
To sum up, we summarized psychosocial factors (including alexithymia, depression, anxiety, negative emotions, perceived stress) and sex hormones (including estrogens, progesterone, androgens, PRL) affecting the susceptibility and development of SLE. Detailed investigations of the roles and effects of psychosocial factors and sex hormones in SLE could provide guidance for possible prevention or treatment measures. Psychosocial factors may impact the progression and susceptibility of SLE. With the removal of psychosocial symptoms, patients with SLE may get better physical treatments. Sex hormones also influence the development and susceptibility of SLE. Some sex hormones (e.g., estrogens, PRL) may act as risk factors for SLE, and some (e.g., progesterone, androgens) may keep protective effects in the development of SLE. The potential mechanisms of them in SLE, such as provoking antibody production and promote the survival of self-reactive B cells, are complicated. Recent research in this regard has offered insight into the biological mechanisms underlying these associations. Thus, practical methods targeting these risk factors based on gaining a greater understanding of the pathogenic mechanism will benefit SLE patients and may provide new therapeutic strategies.
Acknowledgments
The authors wish to appreciate Dr. Haiyan Xiao (Department of Anesthesiology and Perioperative Medicine, Augusta University) and Dr. Jian Zheng (Department of Microbiology, University of Iowa) for assigning this work.
Funding Statement
This work was funded by grants from the National Natural Sciences Foundation of China (81471530, 81202346), Department of Education of Guangdong Province (2017 Key Platform and Scientific Research Project, No. 2017KTSCX077). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yong-zhi Xu, Email: lxyzhi@126.com.
Hua-feng Liu, Email: hf-liu@263.net.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Qingjun Pan conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xiaoqun Chen conceived and designed the experiments, performed the experiments, analyzed the data.
Shuzhen Liao conceived and designed the experiments, performed the experiments, analyzed the data.
Xiaocui Chen performed the experiments, contributed reagents/materials/analysis tools.
Chunfei Zhao performed the experiments, contributed reagents/materials/analysis tools.
Yong-zhi Xu conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hua-feng Liu conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any data or code; this article is a literature review.
References
- Alsowaida et al. (2018).Alsowaida N, Alrasheed M, Mayet A, Alsuwaida A, Omair MA. Medication adherence, depression and disease activity among patients with systemic lupus erythematosus. Lupus. 2018;27(2):327–332. doi: 10.1177/0961203317725585. [DOI] [PubMed] [Google Scholar]
- Altuwaijri et al. (2009).Altuwaijri S, Chuang K-H, Lai K-P, Lai J-J, Lin H-Y, Young FM, Bottaro A, Tsai MY, Zeng W-P, Chang H-C, Yeh S, Chang C. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Molecular Endocrinology. 2009;23(4):444–453. doi: 10.1210/me.2008-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccala et al. (2007).Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature Medicine. 2007;13(5):543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Baechler et al. (2003).Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al. (2016).Bai R, Liu S, Zhao Y, Cheng Y, Li S, Lai A, Xie Z, Xu X, Lu Z, Xu J. Depressive and anxiety disorders in systemic lupus erythematosus patients without major neuropsychiatric manifestations. Journal of Immunology Research. 2016;2016:2829018. doi: 10.1155/2016/2829018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak (2006).Barak Y. The immune system and happiness. Autoimmunity Reviews. 2006;5(8):523–527. doi: 10.1016/j.autrev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Barbasio & Granieri (2013).Barbasio C, Granieri A. Emotion regulation and mental representation of attachment in patients with systemic lupus erythematosus: a study using the adult attachment interview. Journal of Nervous and Mental Disease. 2013;201(4):304–310. doi: 10.1097/NMD.0b013e318288e215. [DOI] [PubMed] [Google Scholar]
- Barbosa et al. (2009).Barbosa F, Mota C, Alves M, Alcantara C, Rossinol B, Patricio P, Barbosa A, Ferreira C. Alexithymia in systemic lupus erythematosus patients. Annals of the New York Academy of Sciences. 2009;1173(1):227–234. doi: 10.1111/j.1749-6632.2009.04640.x. [DOI] [PubMed] [Google Scholar]
- Barbosa et al. (2011).Barbosa F, Mota C, Patricio P, Alcantara C, Ferreira C, Barbosa A. The relationship between alexithymia and psychological factors in systemic lupus erythematosus. Comprehensive Psychiatry. 2011;52(6):754–762. doi: 10.1016/j.comppsych.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Barnhofer et al. (2009).Barnhofer T, Crane C, Hargus E, Amarasinghe M, Winder R, Williams JM. Mindfulness-based cognitive therapy as a treatment for chronic depression: a preliminary study. Behaviour Research and Therapy. 2009;47(5):366–373. doi: 10.1016/j.brat.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi et al. (2015).Bassi N, Luisetto R, Ghirardello A, Gatto M, Valente M, Della Barbera M, Nalotto L, Punzi L, Doria A. 17-β-estradiol affects BLyS serum levels and the nephritogenic autoantibody network accelerating glomerulonephritis in NZB/WF1 mice. Lupus. 2015;24(4–5):382–391. doi: 10.1177/0961203314559636. [DOI] [PubMed] [Google Scholar]
- Beagley & Gockel (2003).Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunology & Medical Microbiology. 2003;38(1):13–22. doi: 10.1016/s0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Bird et al. (2017).Bird AK, Chang M, Barnard J, Goldman BI, Meednu N, Rangel-Moreno J, Anolik JH. Neutrophils slow disease progression in murine lupus via modulation of autoreactive germinal centers. Journal of Immunology. 2017;199(2):458–466. doi: 10.4049/jimmunol.1700354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci-Laudiero et al. (1999).Bracci-Laudiero L, Aloe L, Lundeberg T, Theodorsson E, Stenfors C. Altered levels of neuropeptides characterize the brain of lupus prone mice. Neuroscience Letters. 1999;275(1):57–60. doi: 10.1016/s0304-3940(99)00737-5. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero et al. (1998).Bracci-Laudiero L, Aloe L, Stenfors C, Theodorsson E, Lundeberg T. Development of systemic lupus erythematosus in mice is associated with alteration of neuropeptide concentrations in inflamed kidneys and immunoregulatory organs. Neuroscience Letters. 1998;248(2):97–100. doi: 10.1016/s0304-3940(98)00342-5. [DOI] [PubMed] [Google Scholar]
- Bynoe, Grimaldi & Diamond (2000).Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2703–2708. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey (2012).Choubey D. Interferon-inducible Ifi200-family genes as modifiers of lupus susceptibility. Immunology Letters. 2012;147(1–2):10–17. doi: 10.1016/j.imlet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey et al. (2011).Choubey D, Panchanathan R, Duan X, Liu H, Liu H. Emerging roles for the interferon-inducible p200-family proteins in sex bias in systemic lupus erythematosus. Journal of Interferon & Cytokine Research. 2011;31(12):893–906. doi: 10.1089/jir.2011.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger, Altmann & Prystowsky (1991).Clevenger CV, Altmann SW, Prystowsky MB. Requirement of nuclear prolactin for interleukin-2–stimulated proliferation of T lymphocytes. Science. 1991;253(5015):77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- Clevenger et al. (1990).Clevenger CV, Russell DH, Appasamy PM, Prystowsky MB. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(16):6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza et al. (2015).Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmunity Reviews. 2015;14(3):223–230. doi: 10.1016/j.autrev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Cunningham & Gilkeson (2011).Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clinical Reviews in Allergy & Immunology. 2011;40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- Cunningham et al. (2014).Cunningham MA, Wirth JR, Freeman LR, Boger HA, Granholm AC, Gilkeson GS. Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus. Journal of Neuroinflammation. 2014;11(1):171. doi: 10.1186/s12974-014-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo, Sulli & Straub (2012).Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmunity Reviews. 2012;11(6–7):A460–A464. doi: 10.1016/j.autrev.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Diamond et al. (2006).Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, DeGiorgio LA, Lee J, Triantafyllopoulou A, Cohen-Solal J, Volpe BT. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Advances in Immunology. 2006;89:289–320. doi: 10.1016/s0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- Ding & Zhu (2008).Ding J, Zhu BT. Unique effect of the pregnancy hormone estriol on antigen-induced production of specific antibodies in female BALB/c mice. Steroids. 2008;73(3):289–298. doi: 10.1016/j.steroids.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Dowlati et al. (2010).Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Elera-Fitzcarrald et al. (2017).Elera-Fitzcarrald C, Ugarte-Gil MF, Gamboa-Cardenas RV, Zevallos F, Medina M, Cucho-Venegas JM, Perich-Campos RA, Alfaro-Lozano JL, Rodriguez-Bellido Z, Alarcon GS, Pastor-Asurza CA. Prolactin levels are associated with a pro-inflammatory body mass distribution among women with systemic lupus erythematosus. Lupus. 2017;26(8):808–814. doi: 10.1177/0961203316678673. [DOI] [PubMed] [Google Scholar]
- Fu et al. (2011).Fu Q, Zhao J, Qian X, Wong JL, Kaufman KM, Yu CY, Mok MY, Harley JB, Guthridge JM, Song YW, Cho SK, Bae SC, Grossman JM, Hahn BH, Arnett FC, Shen N, Tsao BP, Hwee Siew Howe and the Tan Tock Seng Hospital Lupus Study Group Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis & Rheumatism. 2011;63(3):749–754. doi: 10.1002/art.30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, Saha & Peeva (2013).Gonzalez J, Saha S, Peeva E. Prolactin rescues and primes autoreactive B cells directly and indirectly through dendritic cells in B6.Sle3 mice. Clinical & Experimental Immunology. 2013;172(2):311–320. doi: 10.1111/cei.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi et al. (2002).Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. Journal of Clinical Investigation. 2002;109(12):1625–1633. doi: 10.1172/jci14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp & Jorgensen (2018).Gubbels Bupp MR, Jorgensen TN. Androgen-induced immunosuppression. Frontiers in Immunology. 2018;9:794. doi: 10.3389/fimmu.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp, Jorgensen & Kotzin (2008).Gubbels Bupp MR, Jorgensen TN, Kotzin BL. Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus. Genes & Immunity. 2008;9(1):47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- Harle et al. (2006).Harle P, Straub RH, Wiest R, Mayer A, Scholmerich J, Atzeni F, Carrabba M, Cutolo M, Sarzi-Puttini P. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Annals of the Rheumatic Diseases. 2006;65(1):51–56. doi: 10.1136/ard.2005.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill et al. (2011).Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Molecular Medicine. 2011;17(3–4):211–220. doi: 10.2119/molmed.2010.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes (2012).Hughes GC. Progesterone and autoimmune disease. Autoimmunity Reviews. 2012;11(6–7):A502–A514. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes & Choubey (2014).Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nature Reviews Rheumatology. 2014;10(12):740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- Hughes, Clark & Wong (2013).Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. Journal of Leukocyte Biology. 2013;93(3):369–375. doi: 10.1189/jlb.1012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes et al. (2009).Hughes GC, Martin D, Zhang K, Hudkins KL, Alpers CE, Clark EA, Elkon KB. Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis & Rheumatism. 2009;60(6):1775–1784. doi: 10.1002/art.24548. [DOI] [PubMed] [Google Scholar]
- Hughes et al. (2008).Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. Journal of Immunology. 2008;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- Jacobson (2000).Jacobson JD. Gonadotropin-releasing hormone and G proteins: potential roles in autoimmunity. Annals of the New York Academy of Sciences. 2000;917(1):809–818. doi: 10.1111/j.1749-6632.2000.tb05446.x. [DOI] [PubMed] [Google Scholar]
- Jacobson (2001).Jacobson JD. Gonadotropin-releasing hormone: potential role in autoimmunity. International Immunopharmacology. 2001;1(6):1077–1083. doi: 10.1016/s1567-5769(01)00038-8. [DOI] [PubMed] [Google Scholar]
- Jacobson et al. (1999).Jacobson JD, Ansari MA, Kinealy M, Muthukrishnan V. Gender-specific exacerbation of murine lupus by gonadotropin-releasing hormone: potential role of Gαq/11*. Endocrinology. 1999;140(8):3429–3437. doi: 10.1210/endo.140.8.6892. [DOI] [PubMed] [Google Scholar]
- Jacobson, Nisula & Steinberg (1994).Jacobson JD, Nisula BC, Steinberg AD. Modulation of the expression of murine lupus by gonadotropin-releasing hormone analogs. Endocrinology. 1994;134(6):2516–2523. doi: 10.1210/endo.134.6.8194477. [DOI] [PubMed] [Google Scholar]
- Jara et al. (2017).Jara LJ, Medina G, Saavedra MA, Vera-Lastra O, Torres-Aguilar H, Navarro C, Vazquez Del Mercado M, Espinoza LR. Prolactin has a pathogenic role in systemic lupus erythematosus. Immunologic Research. 2017;65(2):512–523. doi: 10.1007/s12026-016-8891-x. [DOI] [PubMed] [Google Scholar]
- Karimifar et al. (2013).Karimifar M, Tahmasebi A, Bonakdar ZS, Purajam S. Correlation of serum prolactin levels and disease activity in systematic lupus erythematosus. Rheumatology International. 2013;33(2):511–516. doi: 10.1007/s00296-011-2211-5. [DOI] [PubMed] [Google Scholar]
- Khan & Ansar Ahmed (2016).Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Frontiers in Immunology. 2016;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissick et al. (2014).Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(27):9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats (2012).Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Hormones and Behavior. 2012;62(3):254–262. doi: 10.1016/j.yhbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwerys et al. (2013).Lauwerys BR, Hachulla E, Spertini F, Lazaro E, Jorgensen C, Mariette X, Haelterman E, Grouard-Vogel G, Fanget B, Dhellin O, Vandepapeliere P, Houssiau FA. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis & Rheumatism. 2013;65(2):447–456. doi: 10.1002/art.37785. [DOI] [PubMed] [Google Scholar]
- Li et al. (2010).Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clinical & Experimental Immunology. 2010;159(3):281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2011).Lin HL, Yen JH, Chiou SS, Tsai WC, Ou TT, Wu CC, Liu HW. Estradiol upregulates calcineurin expression via overexpression of estrogen receptor alpha gene in systemic lupus erythematosus. Kaohsiung Journal of Medical Sciences. 2011;27(4):125–131. doi: 10.1016/j.kjms.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai et al. (2010).Mai T, Zan H, Zhang J, Hawkins JS, Xu Z, Casali P. Estrogen receptors bind to and activate the HOXC4/HoxC4 promoter to potentiate HoxC4-mediated activation-induced cytosine deaminase induction, immunoglobulin class switch DNA recombination, and somatic hypermutation. Journal of Biological Chemistry. 2010;285(48):37797–37810. doi: 10.1074/jbc.M110.169086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalka (2003).Matalka KZ. Prolactin enhances production of interferon-γ, interleukin-12, and interleukin-10, but not of tumor necrosis factor-α, in a stimulus-specific manner. Cytokine. 2003;21(4):187–194. doi: 10.1016/s1043-4666(02)00496-9. [DOI] [PubMed] [Google Scholar]
- Matera, Mori & Galetto (2001).Matera L, Mori M, Galetto A. Effect of prolactin on the antigen presenting function of monocyte-derived dendritic cells. Lupus. 2001;10(10):728–734. doi: 10.1191/096120301717164967. [DOI] [PubMed] [Google Scholar]
- Matsunaga et al. (2008).Matsunaga M, Isowa T, Kimura K, Miyakoshi M, Kanayama N, Murakami H, Sato S, Konagaya T, Nogimori T, Fukuyama S, Shinoda J, Yamada J, Ohira H. Associations among central nervous, endocrine, and immune activities when positive emotions are elicited by looking at a favorite person. Brain, Behavior, and Immunity. 2008;22(3):408–417. doi: 10.1016/j.bbi.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Mills et al. (2018).Mills SD, Azizoddin D, Gholizadeh S, Racaza GZ, Nicassio PM. The mediational role of helplessness in psychological outcomes in systemic lupus erythematosus. Lupus. 2018;27(7):1185–1189. doi: 10.1177/0961203317751046. [DOI] [PubMed] [Google Scholar]
- Miyaura & Iwata (2002).Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. Journal of Immunology. 2002;168(3):1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- Mok (2017).Mok CC. Biological and targeted therapies of systemic lupus erythematosus: evidence and the state of the art. Expert Review of Clinical Immunology. 2017;13(7):677–692. doi: 10.1080/1744666x.2017.1323635. [DOI] [PubMed] [Google Scholar]
- Mok (2018).Mok CC. Systemic lupus erythematosus: what should family physicians know in 2018? Hong Kong Medical Journal. 2018;24:501–511. doi: 10.12809/hkmj187319. [DOI] [PubMed] [Google Scholar]
- Moulton (2018).Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Frontiers in Immunology. 2018;9:2279. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, Mastro & Hymer (1990).Mukherjee P, Mastro AM, Hymer WC. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Niewold et al. (2012).Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, Wong AK, Merrill JT, Alarcon-Riquelme ME, Tikly M, Ramsey-Goldman R, Reveille JD, Petri MA, Edberg JC, Kimberly RP, Alarcon GS, Kamen DL, Gilkeson GS, Vyse TJ, James JA, Gaffney PM, Moser KL, Crow MK, Harley JB. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2012;71(3):463–468. doi: 10.1136/annrheumdis-2011-200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, Gu & Kovacs (2001).Olsen NJ, Gu X, Kovacs WJ. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. Journal of Clinical Investigation. 2001;108(11):1697–1704. doi: 10.1172/jci13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach & Shoenfeld (2007).Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmunity Reviews. 2007;6(8):537–542. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Orbach et al. (2012).Orbach H, Zandman-Goddard G, Boaz M, Agmon-Levin N, Amital H, Szekanecz Z, Szucs G, Rovensky J, Kiss E, Doria A, Ghirardello A, Gomez-Arbesu J, Stojanovich L, Ingegnoli F, Meroni PL, Rozman B, Blank M, Shoenfeld Y. Prolactin and autoimmunity: hyperprolactinemia correlates with serositis and anemia in SLE patients. Clinical Reviews in Allergy & Immunology. 2012;42(2):189–198. doi: 10.1007/s12016-011-8256-0. [DOI] [PubMed] [Google Scholar]
- Panchanathan & Choubey (2013).Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Molecular Immunology. 2013;53(1–2):15–23. doi: 10.1016/j.molimm.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan, Liu & Choubey (2013).Panchanathan R, Liu H, Choubey D. Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity. International Immunology. 2013;25(9):521–529. doi: 10.1093/intimm/dxt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan et al. (2009).Panchanathan R, Shen H, Bupp MG, Gould KA, Choubey D. Female and male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. Journal of Immunology. 2009;183(11):7031–7038. doi: 10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan et al. (2010).Panchanathan R, Shen H, Zhang X, Ho S-M, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-α in mice: implications for sex bias in autoimmunity. PLOS ONE. 2010;5(5):e10868. doi: 10.1371/journal.pone.0010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park (2012).Park S-R. Activation-induced cytidine deaminase in B cell immunity and cancers. Immune Network. 2012;12(6):230–239. doi: 10.4110/in.2012.12.6.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks et al. (2017).Parks CG, De Souza Espindola Santos A, Barbhaiya M, Costenbader KH. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Practice & Research Clinical Rheumatology. 2017;31(3):306–320. doi: 10.1016/j.berh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin & Petersen-Mahrt (2009).Pauklin S, Petersen-Mahrt SK. Progesterone inhibits activation-induced deaminase by binding to the promoter. Journal of Immunology. 2009;183(2):1238–1244. doi: 10.4049/jimmunol.0803915. [DOI] [PubMed] [Google Scholar]
- Pauklin et al. (2009).Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. Journal of Experimental Medicine. 2009;206(1):99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell, Galligan & Fish (2012).Pennell LM, Galligan CL, Fish EN. Sex affects immunity. Journal of Autoimmunity. 2012;38(2–3):J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Recalde et al. (2018).Recalde G, Moreno-Sosa T, Yudica F, Quintero CA, Sanchez MB, Jahn GA, Kalergis AM, Mackern-Oberti JP. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmunity Reviews. 2018;17(5):504–512. doi: 10.1016/j.autrev.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Rider et al. (2006).Rider V, Li X, Peterson G, Dawson J, Kimler BF, Abdou NI. Differential expression of estrogen receptors in women with systemic lupus erythematosus. Journal of Rheumatology. 2006;33(6):1093–1101. [PubMed] [Google Scholar]
- Roberts et al. (2018).Roberts AL, Kubzansky LD, Malspeis S, Feldman CH, Costenbader KH. Association of depression with risk of incident systemic lupus erythematosus in women assessed across 2 decades. JAMA Psychiatry. 2018;75(12):1225–1233. doi: 10.1001/jamapsychiatry.2018.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom & Eloranta (2013).Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Current Opinion in Rheumatology. 2013;25(2):248–253. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- Ronnblom, Eloranta & Alm (2006).Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis & Rheumatism. 2006;54(2):408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- Ronnblom & Pascual (2008).Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17(5):394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha et al. (2009).Saha S, Gonzalez J, Rosenfeld G, Keiser H, Peeva E. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis & Rheumatism. 2009;60(6):1743–1752. doi: 10.1002/art.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha et al. (2011).Saha S, Tieng A, Pepeljugoski KP, Zandamn-Goddard G, Peeva E. Prolactin, systemic lupus erythematosus, and autoreactive B cells: lessons learnt from murine models. Clinical Reviews in Allergy & Immunology. 2011;40(1):8–15. doi: 10.1007/s12016-009-8182-6. [DOI] [PubMed] [Google Scholar]
- Sakiani, Olsen & Kovacs (2013).Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nature Reviews Endocrinology. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- Salem (2004).Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Current Drug Target -Inflammation & Allergy. 2004;3(1):97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- Schiepers, Wichers & Maes (2005).Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Scott et al. (2017).Scott JL, Wirth JR, Eudaly J, Ruiz P, Cunningham MA. Complete knockout of estrogen receptor alpha is not directly protective in murine lupus. Clinical Immunology. 2017;183:132–141. doi: 10.1016/j.clim.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly, Boaz & Orbach (2012).Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmunity Reviews. 2012;11(6–7):A465–A470. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2010).Shen H, Panchanathan R, Rajavelu P, Duan X, Gould KA, Choubey D. Gender-dependent expression of murine Irf5 gene: implications for sex bias in autoimmunity. Journal of Molecular Cell Biology. 2010;2(5):284–290. doi: 10.1093/jmcb/mjq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson et al. (2005).Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, Jonsen A, Rantapaa-Dahlqvist S, Moller B, Kere J, Koskenmies S, Widen E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Ronnblom L, Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. American Journal of Human Genetics. 2005;76(3):528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati et al. (2017).Solati K, Mousavi M, Kheiri S, Hasanpour-Dehkordi A. The effectiveness of mindfulness-based cognitive therapy on psychological symptoms and quality of life in systemic lupus erythematosus patients: a randomized controlled trial. Oman Medical Journal. 2017;32(5):378–385. doi: 10.5001/omj.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, Amkraut & Kasper (1974).Solomon GF, Amkraut AA, Kasper P. Immunity, emotions and stress with special reference to the mechanisms of stress effects on the immune system. Psychotherapy and Psychosomatics. 1974;23(1–6):209–217. doi: 10.1159/000286644. [DOI] [PubMed] [Google Scholar]
- Stone et al. (2012).Stone RC, Feng D, Deng J, Singh S, Yang L, Fitzgerald-Bocarsly P, Eloranta ML, Ronnblom L, Barnes BJ. Interferon regulatory factor 5 activation in monocytes of systemic lupus erythematosus patients is triggered by circulating autoantigens independent of type I interferons. Arthritis & Rheumatism. 2012;64(3):788–798. doi: 10.1002/art.33395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland et al. (2012).Strickland FM, Hewagama A, Lu Q, Wu A, Hinderer R, Webb R, Johnson K, Sawalha AH, Delaney C, Yung R, Richardson BC. Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus. Journal of Autoimmunity. 2012;38(2–3):J135–J143. doi: 10.1016/j.jaut.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor & Gould (2017).Tabor DE, Gould KA. Estrogen receptor alpha promotes lupus in (NZB×NZW)F1 mice in a B cell intrinsic manner. Clinical Immunology. 2017;174:41–52. doi: 10.1016/j.clim.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait, Butts & Sternberg (2008).Tait AS, Butts CL, Sternberg EM. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. Journal of Leukocyte Biology. 2008;84(4):924–931. doi: 10.1189/jlb.0208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Peeva & Zandman-Goddard (2015).Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system—a spotlight on the role of progestogens. Autoimmunity Reviews. 2015;14(6):536–542. doi: 10.1016/j.autrev.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Tedeschi, Bermas & Costenbader (2013).Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clinical Immunology. 2013;149(2):211–218. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Tsur et al. (2015).Tsur A, Hughes GC, Shoenfeld Y, Carp H. Interdisciplinary exchange of ideas: progestagens for autoimmunity, biologics for pregnancy complications. Immunologic Research. 2015;61(1–2):31–34. doi: 10.1007/s12026-014-8621-1. [DOI] [PubMed] [Google Scholar]
- Vadacca et al. (2008).Vadacca M, Bruni R, Cacciapaglia F, Serino F, Arcarese L, Buzzulini F, Coppolino G, Rigon A, Terminio N, Afeltra A. Alexithymia and immunoendocrine parameters in patients affected by systemic lupus erythematosus and rheumatoid arthritis. Reumatismo. 2008;60(1):50–56. doi: 10.4081/reumatismo.2008.50. [DOI] [PubMed] [Google Scholar]
- Vadacca et al. (2014).Vadacca M, Bruni R, Terminio N, Sambataro G, Margiotta D, Serino FM, Afeltra A. Alexithymia, mood states and pain experience in systemic lupus erythematosus and rheumatoid arthritis. Clinical Rheumatology. 2014;33(10):1443–1450. doi: 10.1007/s10067-014-2593-3. [DOI] [PubMed] [Google Scholar]
- Walker & Jacobson (2000).Walker SE, Jacobson JD. Roles of prolactin and gonadotropin-releasing hormone in rheumatic diseases. Rheumatic Disease Clinics of North America. 2000;26(4):713–736. doi: 10.1016/s0889-857x(05)70166-6. [DOI] [PubMed] [Google Scholar]
- Walters et al. (2009).Walters E, Rider V, Abdou NI, Greenwell C, Svojanovsky S, Smith P, Kimler BF. Estradiol targets T cell signaling pathways in human systemic lupus. Clinical Immunology. 2009;133(3):428–436. doi: 10.1016/j.clim.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang C, Sandling JK, Hagberg N, Berggren O, Sigurdsson S, Karlberg O, Ronnblom L, Eloranta ML, Syvanen A-C. Genome-wide profiling of target genes for the systemic lupus erythematosus-associated transcription factors IRF5 and STAT4. Annals of the Rheumatic Diseases. 2013;72(1):96–103. doi: 10.1136/annrheumdis-2012-201364. [DOI] [PubMed] [Google Scholar]
- Watson et al. (2012).Watson P, Storbeck J, Mattis P, Mackay M. Cognitive and emotional abnormalities in systemic lupus erythematosus: evidence for amygdala dysfunction. Neuropsychology Review. 2012;22(3):252–270. doi: 10.1007/s11065-012-9213-2. [DOI] [PubMed] [Google Scholar]
- Yakimchuk, Jondal & Okret (2013).Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Molecular and Cellular Endocrinology. 2013;375(1–2):121–129. doi: 10.1016/j.mce.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang L, Fu T, Yin R, Zhang Q, Shen B. Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):70. doi: 10.1186/s12888-017-1234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2010).Zhang Y, Saha S, Rosenfeld G, Gonzalez J, Pepeljugoski KP, Peeva E. Raloxifene modulates estrogen-mediated B cell autoreactivity in NZB/W F1 mice. Journal of Rheumatology. 2010;37(8):1646–1657. doi: 10.3899/jrheum.090911. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2016).Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, Starmer J, Wilson EM, Su MA. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nature Communications. 2016;7(1):11350. doi: 10.1038/ncomms11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any data or code; this article is a literature review.