Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia (original) (raw)
. Author manuscript; available in PMC: 2014 Aug 1.
Abstract
The origin of inflammation in psychiatric disorders is not well understood. The translocation of commensal microbiota across the gastrointestinal barrier can result in a persistent state of low-grade immune activation and/or inflammation. We measured serological surrogate markers of bacterial translocation (soluble CD14 (sCD14) and lipopolysaccharide binding protein (LBP)) in two psychiatric cohorts and compared these levels to C-reactive protein (CRP), body mass index (BMI), and food-related and autoimmune antibodies. The two cohorts were composed of the following: (1) n=141 schizophrenia, n=75 bipolar disorder, n=78 controls; (2) n=78 antipsychotic-naïve first-episode schizophrenia, n=38 medicated first-episode schizophrenia. sCD14 seropositivity conferred a 3.1-fold increased odds of association with schizophrenia (multivariate regressions, OR=3.09, p<0.0001) compared to controls. Case-control differences in sCD14 were not matched by LBP. Quantitative levels of LBP, but not sCD14, correlated with BMI in schizophrenia (R2=0.21, p<0.0001). sCD14 and LBP also exhibited some congruency in schizophrenia with both significantly correlated with CRP (R2=0.26-0.27, p<0.0001) and elevated in females compared to males (p<0.01). Antipsychotic treatment generally did not impact sCD14 or LBP levels except for significant correlations, especially sCD14, with gluten antibodies in antipsychotic-naïve schizophrenia (R2=0.27, p<0.0001). In bipolar disorder, sCD14 levels were significantly correlated with anti-tissue transglutaminase IgG (R2=0.37, p<0.001). In conclusion, these bacterial translocation markers produced discordant and complex patterns of activity, a finding that may reflect an imbalanced, activated innate immune state. Whereas both markers may upregulate following systemic exposure to Gram-negative bacteria, non-lipopolysaccharide-based monocyte activation, autoimmunity and metabolic dysfunction may also contribute to the observed marker profiles.
Keywords: gut, microbiome, diet, metabolic syndrome, mental illness, psychosis, macrophage
1. Introduction
Chronic inflammation is a risk factor intrinsically linked to cardiovascular disease, type 2 diabetes, insulin resistance and obesity (Berg and Scherer, 2005; Gregor and Hotamisligil, 2011; Hansson, 2005; Hotamisligil, 2006). Inflammatory abnormalities in systemic circulation and in the central nervous system are increasingly reported in psychiatric diseases such as schizophrenia and bipolar disorder (Dickerson et al., 2013; Drexhage et al., 2010; Fillman et al., 2013; Gibney and Drexhage, 2013; Leonard et al., 2012; Miller et al., 2011; Miller et al., 2012; Muller et al., 2012a; Padmos et al., 2009; Severance et al., 2012b). The inflammation source is not known but may be inherent to the disease or may result from antipsychotic-related metabolic disturbances (Drexhage et al., 2010; Leonard et al., 2012; Miller et al., 2011; Miller et al., 2012; Muller et al., 2012a; Severance et al., 2012a; Severance et al., 2012b; Steiner et al., 2012). A chronic inflammatory state predisposes the bearer to a heightened risk of comorbidities that compromise general health and quality of life; therefore, it is important to understand the source of abnormal inflammation and implement corrective treatments.
Several risk factors for the development of schizophrenia (inflammation, Toxoplasma gondii exposures, gluten sensitivity) can be linked through a common origin in the gastrointestinal (GI) tract (Dickerson et al., 2011; Dickerson et al., 2010; Dickerson et al., 2012; Niebuhr et al., 2011; Samaroo et al., 2010; Severance et al., 2012a; Severance et al., 2010a; Severance et al., 2010b; Severance et al., 2012c). The innate immune molecule, complement C1q, forms immune complexes with food antigens at increased rates in individuals with schizophrenia (Severance et al., 2012b). Exposure to the gut pathogen, T. gondii, results in elevated antibodies to dietary gluten in both humans and experimentally infected animals (Severance et al., 2012a; Severance et al., 2012c). Food-derived antibodies in schizophrenia coincide with antibody levels to the commensal fungus, Saccharomyces cerevisiae, a marker of microbial translocation and GI inflammation used to help diagnose Crohn’s disease (Severance et al., 2012a).
If the GI barrier is differentially compromised in schizophrenia, we would expect that increased levels of gut-dwelling commensal enteric bacteria are exposed to systemic circulation, a process known as bacterial translocation (Brenchley et al., 2006; Sandler and Douek, 2012). Heightened rates of bacterial translocation are reported in individuals with unipolar depression compared to controls (Maes et al., 2008; Maes et al., 2012; Maes et al., 2013). Here, we measured two markers of bacteria translocation, soluble CD14 (sCD14) and lipopolysaccharide (LPS) binding protein (LBP), in serum samples from one cohort of individuals with schizophrenia, bipolar disorder and non-psychiatric controls and from a second cohort of individuals with first-episode schizophrenia who were antipsychotic-naïve or who had received antipsychotic medication.
2. Experimental/ Materials and methods
2.1 Study participants
2.1.1 Cohort 1 - Sheppard Pratt Health System, Baltimore, MD, USA
One hundred and forty one individuals with schizophrenia, 75 individuals with bipolar disorder and 78 individuals with no history of psychiatric disorders were recruited. Methods for identifying and characterizing individuals of diagnostic groups according to criteria defined by DSM-IV have been previously described (Dickerson et al., 2011; Dickerson et al., 2013; Severance et al., 2012a; Severance et al., 2010b). Individuals with schizophrenia had a DSM-IV diagnosis (DSM-IV, 1994) of schizophrenia, schizophreniform disorder or schizoaffective disorder and were currently receiving antipsychotic medications. Individuals with bipolar disorder had a DSM-IV diagnosis of type I or type II bipolar disorder or bipolar disorder not otherwise specified. For both groups, inclusion criteria required an age between 18-65. Individuals without a history of psychiatric disorder were recruited from posted announcements and were screened to rule out current or past psychiatric disorders with the Structured Clinical Interview for DSM-IV Axis I Disorders Non-Patient Edition (First, 1998). Control participants were between the ages of 20 and 60, inclusive.
Exclusion criteria included the following: any history of intravenous substance abuse; mental retardation; clinically significant medical disorder that would affect cognitive performance. For psychiatric participants, having a primary diagnosis of substance abuse or dependence was also an exclusion criterion. Basic demographic and clinical data of the three cohort 1 study populations are shown in Table 1.
Table 1.
Demographic and other clinical information of the two study cohorts.
| n | AgeMean years±SEMa | Femalen(%) | AfricanAmericann(%) | BMI | Smokingstatusn(%) | |
| Cohort 1 - Sheppard Pratt, Baltimore, MD, USA | ||||||
| Controls | 78 | 34.4±1.4 | 56 (71.8) | 31 (39.7) | - | 14 (17.9) |
| Schizophrenia | 141 | 40.36±0.9b | 56 (39.7)d | 65 (46.1) | 30.54±0.61 | 83 (58.9)g |
| Bipolar disorder | 75 | 35.1±1.4 | 52 (69.3) | 18 (24.0)e | 27.17±0.77f | 26 (34.7)h |
| Cohort 2 - University of Cologne, Cologne, Germany | ||||||
| First-episode schizophrenia - medicated | 38 | 36.4±2.3 | 20 (52.6) | - | - | - |
| First-episode schizophrenia - unmedicated | 78 | 29.9±1.1c | 31 (39.7) | - | - | - |
Blood samples were obtained by venipuncture, and plasma and serum separated and assessed for biological markers in the assays described below.
The studies were approved by the Institutional Review Boards (IRB) of the Sheppard Pratt Health System and the Johns Hopkins Medical Institution following established guidelines. All participants provided written informed consent after study procedures were explained. The work described was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.1.2 Cohort 2 - University of Cologne, Cologne, Germany
Methods for identifying and characterizing individuals with a first episode of schizophrenia according to criteria defined by DSM-IV have been previously described (Leweke et al., 2004). Seventy-eight patients were antipsychotic-naïve and 38 were currently receiving antipsychotic medication. Demographic data regarding age and sex are listed in Table 1. Patients were excluded from the study if they had a relevant comorbidity such as heart disease, liver cirrhosis, known immune disease (such as multiple sclerosis), or a history of substance dependence. The region from which patients were recruited was generally homogenous regarding socioeconomic characteristics. Informed written consent was obtained from all study participants. Protocols for sample collection and analyses were approved by the ethics committee at the University of Cologne in accordance with the Declaration of Helsinki.
2.2 Laboratory procedures
sCD14 and LBP levels were measured according to the manufacturer’s protocol using commercially available kits (Human sCD14 Quantikine ELISA kit, R&D Systems, Minneapolis, MN, U.S.A.; Multispecies Lipopolysaccharide Binding Protein ELISA Kit, Cell Sciences, Canton, MA, U.S.A.). Serum dilutions were 1:200 for sCD14 and 1:300 for LBP. Intra-assay variation coefficients were 5.5% for sCD14 and 6.1% for LBP. Marker levels were compared to previously generated data for wheat gluten IgG, bovine casein IgG, C-reactive protein (CRP) and tissue transglutaminase (tTG) IgG (Dickerson et al., 2011; Dickerson et al., 2010; Dickerson et al., 2013; Severance et al., 2012a; Severance et al., 2010a; Severance et al., 2010b).
2.3 Statistical analyses
sCD14 and LBP concentrations for each sample were calculated based on standard curves constructed with assay kit standards run for each assay plate. Quantitative levels of each marker were compared between groups within each cohort using ANOVAs, post-hoc Sidak correction and two-tailed t-tests. In cohort 1, all individuals with schizophrenia received antipsychotic drug treatment. To test for antipsychotic effects on markers in this cohort, we compared marker levels in the bipolar disorder group where 57 individuals received antipsychotics and 18 were antipsychotic-naïve. Cohort 2 inter-group analyses addressed the impact of antipsychotic treatment on marker levels in individuals with first-episode schizophrenia. Groups were also subdivided according to sex so that sex-specific differences in quantitative marker levels could be identified.
Odds ratios for disease association were calculated in cohort 1 using multivariate logistic regression models corrected for age, sex, race and smoking status. Marker seropositivity was established based on 90% control values.
Multiple linear regressions corrected for age, sex, race and smoking status were implemented to identify inter-correlations of marker levels and antibodies to gluten and casein in both cohorts. Cohort 1 data were also analyzed for correlations of markers with anti-tTG IgG, CRP and BMI scores, although data available for the latter two variables were limited (corresponding sample sizes are thus listed in the results text). For cohort 2, marker inter-correlations were compared in antipsychotic-positive and antipsychotic-naive individuals with first-episode schizophrenia using multiple linear regressions corrected for age and sex. For both cohorts, regression values that equaled or exceeded 0.15 and p values less than 0.05 were considered significant. For both cohorts, multivariate comparisons included assay plate designations to control for plate-to-plate variation. Statistical analyses were performed with STATA version 12 (STATA Corp LP, College Station, Texas, U.S.A.).
3. Results
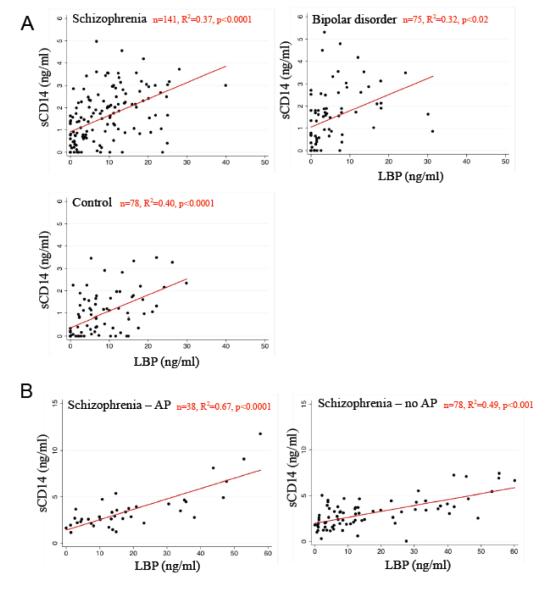
In multivariate regressions, quantitative levels of sCD14 and LBP were significantly inter-correlated in both cohorts in all diagnostic and treatment groups, (Figure 1; R2=0.32-0.67, p<0.0001-0.02).
Figure 1.
Markers of bacterial translocation, sCD14 and LBP, were inter-correlated. A. sCD14 and LBP marker correlations in cohort 1 based in Baltimore, MD, U.S.A. are shown. Multiple linear regressions included age, gender, race, smoking status, and assay plate designation. B. sCD14 and LBP marker correlations in cohort 2 based in Cologne, Germany, are shown. Multiple linear regressions included age, gender, and assay plate designation. AP refers to antipsychotic. [For color reproduction]
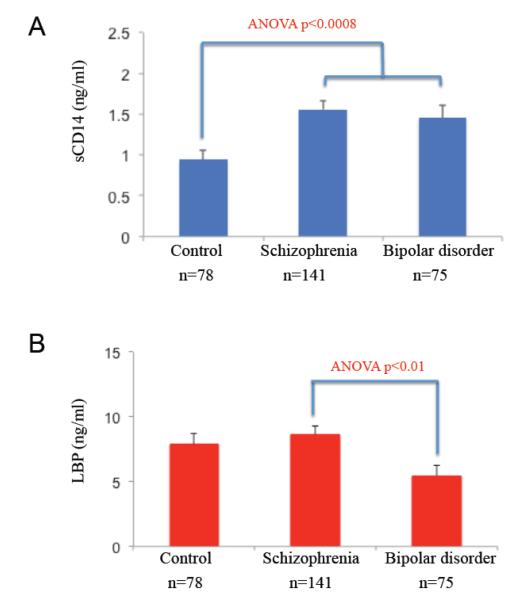
In comparisons between diagnostic groups in cohort 1, sCD14 levels were significantly greater in schizophrenia and bipolar disorder compared to controls (Figure 2; ANOVA, F=7.36, p<0.0008; Sidak post-hoc, schizophrenia p<0.001, bipolar disorder p<0.02). sCD14 seropositivity was associated with a significant odds ratio (OR) of 3.09 for schizophrenia (CI=1.23-7.80, p<0.0001) and a trend towards a significant OR (2.49) for bipolar disorder (CI=0.95-6.52, p<0.065) compared to controls. Levels of LBP and LBP seropositivities were not significantly different between cases and controls, although levels in the bipolar disorder group were significantly decreased compared to levels in the schizophrenia group (ANOVA, F=4.38, p<0.01, Sidak post-hoc, p<0.01).
Figure 2.
sCD14 and LBP levels in cohort 1 cases compared to controls. A. sCD14 levels were elevated in schizophrenia and bipolar disorder compared to controls. B. LBP levels were not different between cases and controls, but levels in schizophrenia were higher than levels in bipolar disorder. [For color reproduction]
To evaluate the effects of antipsychotic treatment on marker levels, we compared sCD14 and LBP levels between (1) cohort 1 individuals with bipolar disorder who were receiving antipsychotics (n=57) and those who were not (n=18), and (2) cohort 2 individuals with first-episode schizophrenia who were antipsychotic-naïve (n=78) and who were not (n=38). In neither cohort were there significant differences in levels of either marker between treatment groups.
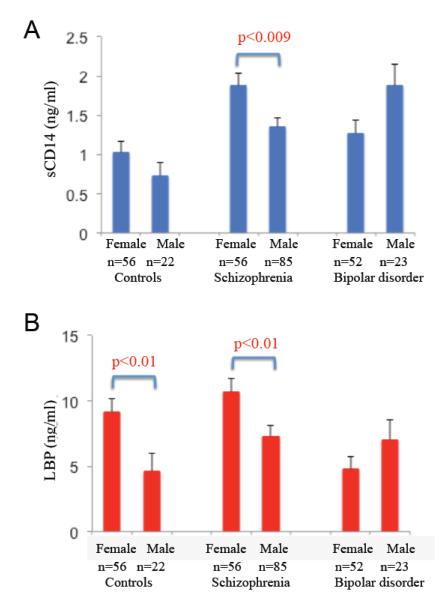
Differences in the levels of sCD14 and LBP were found to be sex-specific in cohort 1 (Figure 3). Females with schizophrenia had greater levels of sCD14 and LBP than male counterparts (two-tailed t-tests, sCD14, t=2.66, p<0.009; LBP, t=2.54, p<0.01). LBP levels in female controls were also significantly greater than levels in male controls (two-tailed t-tests, t=2.58, p<0.01). No sex-specific difference in the levels of these markers was observed in the bipolar group or between or within the medicated and unmedicated individuals in cohort 2.
Figure 3.
sCD14 and LBP exhibited sex-specific patterns. A. sCD14 levels were elevated in female individuals with schizophrenia compared to males. B. LBP levels were elevated in females compared to males in both the control and schizophrenia groups. [For color reproduction]
In both cohorts, we measured associations of the markers with antibodies to bovine casein and wheat gluten. In cohort 1, neither sCD14 nor LBP were correlated with casein or gluten antibodies. In cohort 2, sCD14 and LBP were significantly correlated with antibodies to gluten in the antipsychotic-naïve group (Table 2; sCD14, R2=0.27, coefficient=6.74, CI=3.19-10.29, p<0.0001; LBP, R2=0.16, coefficient=50.86, CI=11.71-90.01, p<0.01). Antibodies to casein were not significantly associated with sCD14 or LBP levels in either the treated or antipsychotic-naïve cohort 2 groups.
Table 2.
Associations of sCD14 and LBP with gluten IgG, CRP, BMI scores and tTG IgG.
| Gluten IgG | CRP | BMI score | tTG IgG | |||||
| n | R2, coefficient95th% CI, p-value | n | R2, coefficient95th% CI, p-value | n | R2, coefficient95th% CI, p-value | R2, coefficient95th% CI, p-value | ||
| sCD14 | ||||||||
| Cohort 1 - Sheppard Pratt, Baltimore, MD, U.S.A. | ||||||||
| Control | 78 | 0.19, <0.008−0.06-0.04, ns | 55 | 0.07, −0.24−0.74-0.26, ns | - | - | 78 | 0.30, −1.09−2.37-0.20, ns |
| Schizophrenia | 141 | 0.10, 0.020.002-0.04, ns | 99 | 0.26, 0.27 a 0.04-0.51, 0.0001 | 121 | 0.28, <0.02-0.05-0.006, ns | 141 | 0.24, 0.04−1.23-1.30, ns |
| Bipolar disorder | 75 | 0.10, 0.030.002-0.07, ns | 56 | 0.38, 0.23−0.36-0.81, ns | 74 | 0.25, 0.02−0.02-0.06, ns | 75 | 0.37, 8.6 3.69-13.51, 0.001 |
| Cohort 2 - University of Cologne, Cologne, Germany | ||||||||
| First-episode schizophrenia - unmedicated | 78 | 0.27, 6.74 3.19-10.29, 0.0001 | - | - | - | - | - | - |
| First-episode schizophrenia - medicated | 38 | 0.04, 1.14−3.16-5.45, ns | - | - | - | - | - | - |
| LBP | ||||||||
| Cohort 1 - Sheppard Pratt, Baltimore, MD, U.S.A. | ||||||||
| Control | 78 | 0.19, <0.00004−0.007-0.007, ns | 55 | 0.14, 3.23−1.05-7.52, ns | - | - | 78 | 0.23, −6.39−16.47-3.69, ns |
| Schizophrenia | 141 | 0.08, 0.002−0.0006-0.005, ns | 99 | 0.27, 3.35 1.68-5.02, 0.0001 | 121 | 0.21, 0.40 0.19-0.62, 0.0001 | 141 | 0.13, 3.81−5.25-12.87, ns |
| Bipolar disorder | 75 | 0.06, 0.003−0.003-0.009, ns | 56 | 0.26, 2.45−0.29-5.19, ns | 74 | 0.28, <0.05−0.27-0.17, ns | 75 | 0.28, −8.61−36.62-19.40, ns |
| Cohort 2 - University of Cologne, Cologne, Germany | ||||||||
| First-episode schizophrenia - unmedicated | 78 | 0.16, 50.86 11.71-90.01, 0.01 | - | - | - | - | - | - |
| First-episode schizophrenia - medicated | 38 | 0.02, 6.59−24.38-37-56, ns | - | - | - | - | - | - |
We tested associations of these markers with other known indices of inflammation: CRP, BMI score and smoking status. We found that levels of sCD14 and LBP were both significantly correlated with CRP in schizophrenia (Table 2; n=99, sCD14, R2=0.26, coefficient=0.27, CI=0.04-0.51, p<0.0001; LBP, R2=0.27, coefficient=3.35, CI=1.68-5.02, p<0.0001) but not in bipolar disorder (n=56) or in controls (n=55). Levels of LBP were also significantly correlated with BMI in individuals with schizophrenia (n=121, R2=0.21, coefficient=0.40, CI=0.19-0.62, p<0.0001) but not in bipolar disorder (n=74). BMI was significantly elevated in schizophrenia compared to bipolar disorder (schizophrenia n=121, 30.54+0.61; bipolar disorder n=74, 27.17+0.77, two-tailed t-test t= −3.43, p<0.0007). BMI data were not available for controls. sCD14 levels were not significantly correlated with BMI. Smoking status was not significantly associated with either sCD14 or LBP levels in any of the diagnostic groups.
Finally, we evaluated sCD14 and LBP levels for a possible autoimmune connection by examining correlations of the markers with celiac disease-associated anti-tTG IgG antibodies. Neither marker was significantly associated with anti-tTG antibodies in schizophrenia, but this autoimmune target was significantly correlated with sCD14 in bipolar disorder (n=75, R2=0.37, coefficient=8.6, CI-3.69-13.51, p<0.001).
4. Discussion
We found that the relationship between the bacterial translocation markers used in this study was complex. sCD14 and LBP levels were significantly inter-correlated, as consistent with their coordinated roles in activating the innate immune system (Figure 4). These markers were also both significantly correlated with CRP in individuals with schizophrenia, suggesting a common pathway of associated inflammation. In spite of these marker congruencies, a main outcome of our study was the detection of markedly elevated sCD14 in schizophrenia (multivariate OR=3.09, p<0.0001) compared to controls, which was not matched by increased LBP. Conversely, we also found significant correlations of LBP with BMI scores in schizophrenia, a pattern that was not matched by sCD14. Our analyses uncovered associations particularly of sCD14 with gluten antibodies in antipsychotic-naïve schizophrenia and with anti-tTG IgG antibodies in bipolar disorder. Whereas bacterial translocation might contribute in part to psychiatric disorder-associated inflammation, other forms of innate immune system dysregulation inherent to the disease may also explain our results.
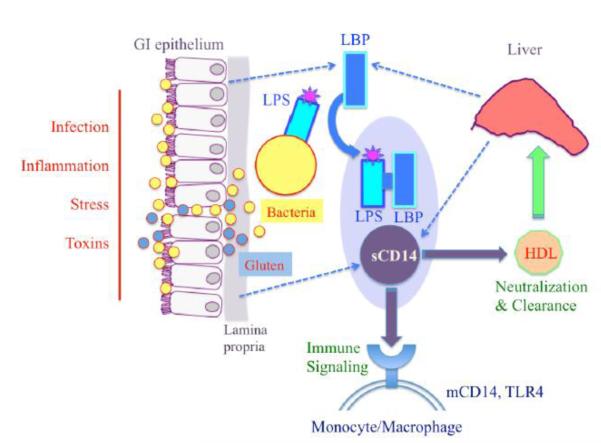
Figure 4.
Overview of coordinated activation of sCD14 and LBP during bacterial translocation. Following episodes of increased GI permeability, enteric bacteria may be exposed to the immune cells of the lamina propria and to systemic circulation. LBP interacts directly with the LPS endotoxin of Gram-negative bacteria and transports LPS monomers to sCD14 and CD14 bound to macrophage membranes. sCD14 is manufactured by immune cells of the GI mucosa, whereas LBP is synthesized by GI epithelial cells. Both markers are also produced by the liver (Heumann and Roger, 2002; Kitchens and Thompson, 2005; Miyake, 2004; Sandler and Douek, 2012; Stehle et al., 2012). Dietary gluten may impact components of these pathways and based on our study results is included in a hypothetical role in this diagram. [For color reproduction]
The discordant patterns of sCD14 and LBP in our study implicate that sCD14 upregulation in schizophrenia could be a function of monocyte activation that is independent from LPS activity. Discrepancies between these markers have been detected in HIV patients, particularly during disease stages when immune marker profiles were generally healthy and sCD14 activation was thought to more accurately reflect systemic activation by HIV rather than by LPS (Romero-Sanchez et al., 2012). In schizophrenia and other psychoses, abnormal monocyte or macrophage activation has been reported previously and can represent a state of innate immune hyper-reactivity (Beumer et al., 2012a; Beumer et al., 2012b; Drexhage et al., 2010; Drexhage et al., 2011; Miller et al., 2012; Muller et al., 2012b). An imbalanced innate immune system can also be a function of active autoimmunity, and higher prevalences of autoimmune disorders are reported in individuals with schizophrenia and their parents compared to controls (Eaton et al., 2006; Gibney and Drexhage, 2013). The connection between schizophrenia and the autoimmune disorder, celiac disease, is based on a long history of associations (Cascella et al., 2011; Dickerson et al., 2010; Dohan, 1966, 1970, 1980; Graff and Handford, 1961; Jackson et al., 2012b; Samaroo et al., 2010). Here, sCD14 was significantly correlated with anti-gluten IgG in the antipsychotic-naïve schizophrenia group only and with anti-tTG antibodies in the bipolar disorder group only, findings that implicate a role of sCD14 in autoimmune processes in both diseases.
The low-grade inflammation observed in mental illnesses may reflect pathways related to metabolic syndrome and use of antipsychotic medications (Beumer et al., 2012a; Drexhage et al., 2010; Drexhage et al., 2011; Leonard et al., 2012; Miller et al., 2012; Steiner et al., 2012). LBP is a known surrogate measure of chronic inflammation originating from metabolic disturbances such as obesity and insulin resistance (Gonzalez-Quintela et al., 2013; Sun et al., 2010). The correlations of LBP with BMI in the schizophrenia group support that at least part of the inflammation measured in this disease has a metabolic root which may be mediated by signaling pathways in adipose tissue (Gregor and Hotamisligil, 2011; Hotamisligil, 2006). Low-grade, chronic inflammation stemming from metabolic issues associated with the female sex is also consistent with our results. Our findings thus have implications for individuals with psychiatric disorders regarding comorbid inflammation-based medical conditions such as cardiovascular disease, obesity, diabetes and cancer. Premature mortality differentially afflicts individuals with psychiatric disorders compared to controls, with ischemic heart disease and cancer representing some of the leading causes of death (Crump et al., 2013; Hennekens et al., 2005; Kilbourne et al., 2009; Laursen et al., 2012; Saha et al., 2007; Suvisaari et al., 2013).
Univariate sCD14 levels were significantly elevated in bipolar disorder, although multivariate analyses failed to reach statistical significance. As noted earlier, associations of sCD14 with the autoimmune marker, tTG, in multivariate regressions were significant in bipolar disorder, and lend support to a role of autoimmunity in this disease (Eaton et al., 2010). LBP levels were low in bipolar disorder compared to schizophrenia, and this finding is consistent with a generally low average BMI score in the bipolar disorder group. Although LBP and BMI scores were not statistically significantly correlated in bipolar disorder, there is likely a minimum value range that must be exceeded in order for associations of these two variables to be measurable. It is also possible in bipolar disorder that other drugs used to treat mood disorders including lithium and valproate may affect sCD14 and LBP levels.
In assessments of antipsychotic effects, we found no differences in sCD14 and LBP levels between the treatment groups in our cohorts. However, we cannot exclude the possibility that antipsychotics might modulate these or other inflammatory markers, particularly with respect to side effects that contribute to metabolic dysfunction (Drexhage et al., 2010; Leonard et al., 2012; Miller et al., 2012; Steiner et al., 2012). As noted earlier, we uncovered an interesting association of gluten antibodies particularly with sCD14 in individuals who were antipsychotic-naïve, thus supporting the possibility of a state of inflammation specific to schizophrenia that is independent of pharmacological treatment. In experimental models, gluten activates monocytes (Cinova et al., 2007; Jelinkova et al., 2004; Thomas et al., 2006; Tlaskalova-Hogenova et al., 2005), and we include this activation pathway in Figure 4 in a hypothetical context. Results from in vitro studies also suggest that gluten-derived peptides are involved in intestinal permeability processes by inducing zonulin release from intestinal epithelial cells (Clemente et al., 2003; Lammers et al., 2008; Thomas et al., 2006). Preliminary reports in humans and mouse models indicate that a gluten-free diet may help to reduce obesity, inflammation and insulin resistance as well as exert positive behavioral changes and symptom improvement in neuropsychiatric disorders (Garcia-Manzanares et al., 2011; Jackson et al., 2012a; Reichelt and Knivsberg, 2009; Soares et al., 2012; Whiteley et al., 2010; Whiteley et al., 2012).
Our data interpretation is limited by several confounding factors. The two cohorts were recruited with different study designs and thus have different exclusion criteria. The cohorts vary in terms of inclusion of immunological variables such as a history of immune disorders, recent infection and use of anti-inflammatory agents or other immune-modulatory drugs. Furthermore, fasting conditions or time of blood draw were not standardized, which may not significantly impact IgG antibodies but may affect other immune or inflammatory markers. Additionally, a history of drug abuse was based on self-reporting rather than a urine drug screen.
In conclusion, schizophrenia-associated inflammation likely has multiple origins and facilitators. Monocyte activation may reflect inherent aberrant immune cell activities in schizophrenia, autoimmunities, bacterial translocation or other immune activators such as food antigens. Furthermore, concurrent low-grade inflammation could originate with, be fueled by or result in metabolic disturbances. Given the propensity for comorbid, inflammation-based risk factors in schizophrenia and the role of inflammation in serious health conditions including cardiovascular disease, diabetes and cancer, supplemental treatment strategies that target inflammation or correction of gut microbiota dysbioses warrant increased evaluation for effectiveness in psychiatric disorders.
Acknowledgments
We thank Ruby Pittman for technical assistance and Ann Cusic for administrative support.
Role of funding source This work was supported by the Brain and Behavior Research Foundation (formerly NARSAD) where Dr. Severance is a Scott-Gentle Foundation Young Investigator; by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268); and by the Stanley Medical Research Institute. These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
sCD14
soluble CD14
LBP
lipopolysaccharide binding protein
LPS
lipopolysaccharide
CRP
C-reactive protein
BMI
body mass index
GI
gastrointestinal
tTG
tissue transglutaminase
Footnotes
Contributors Drs. Severance and Yolken designed the study with input from Drs. Dickerson, and Leweke. All authors collected and/or analyzed data. Dr. Severance wrote the first draft of the manuscript. All authors approved the final manuscript.
Conflict of Interest Robert Yolken is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. None of the other authors report any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012a;37(12):1901–1911. doi: 10.1016/j.psyneuen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012b;92(5):959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37(1):94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinova J, Palova-Jelinkova L, Smythies LE, Cerna M, Pecharova B, Dvorak M, Fruhauf P, Tlaskalova-Hogenova H, Smith PD, Tuckova L. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27(2):201–209. doi: 10.1007/s10875-006-9061-z. [DOI] [PubMed] [Google Scholar]
- Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324–333. doi: 10.1176/appi.ajp.2012.12050599. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in bipolar disorder. Bipolar Disord. 2011;13(1):52–58. doi: 10.1111/j.1399-5618.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68(1):100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yang S, Yolken R. C-reactive protein is elevated in schizophrenia. Schizophr Res. 2013;143(1):198–202. doi: 10.1016/j.schres.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Markers of gluten sensitivity in acute mania: A longitudinal study. Psychiatry Res. 2012;196(1):68–71. doi: 10.1016/j.psychres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Wartime changes in hospital admissions for schizophrenia. A comparison of admission for schizophrenia and other psychoses in six countries during World War II. Acta Psychiatr Scand. 1966;42(1):1–23. doi: 10.1111/j.1600-0447.1966.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Coeliac disease and schizophrenia. Lancet. 1970;1(7652):897–898. doi: 10.1016/s0140-6736(70)91729-0. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Celiac disease and schizophrenia. N Engl J Med. 1980;302(22):1262. doi: 10.1056/NEJM198005293022216. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Weigelt K, van Beveren N, Cohen D, Versnel MA, Nolen WA, Drexhage HA. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int Rev Neurobiol. 2011;101:169–201. doi: 10.1016/B978-0-12-387718-5.00007-9. [DOI] [PubMed] [Google Scholar]
- DSM-IV . Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, Mortensen PB. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163(3):521–528. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12(6):638–646. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Non-patient Edition (SCID I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- Garcia-Manzanares A, Lucendo AJ, Gonzalez-Castillo S, Moreno-Fernandez J. Resolution of metabolic syndrome after following a gluten free diet in an adult woman diagnosed with celiac disease. World J Gastrointest Pathophysiol. 2011;2(3):49–52. doi: 10.4291/wjgp.v2.i3.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney SM, Drexhage HA. Evidence for a Dysregulated Immune System in the Etiology of Psychiatric Disorders. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9462-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of Serum Concentrations of Lipopolysaccharide-Binding Protein (LBP) in the Adult Population: The Role of Obesity. PLoS One. 2013;8(1):e54600. doi: 10.1371/journal.pone.0054600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff H, Handford A. Celiac syndrome in the case histories of five schizophrenics. Psychiatr Q. 1961;35:306–313. doi: 10.1007/BF01566581. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323(1-2):59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jackson J, Eaton W, Cascella N, Fasano A, Warfel D, Feldman S, Richardson C, Vyas G, Linthicum J, Santora D, Warren KR, Carpenter WT, Jr., Kelly DL. A glutenfree diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophr Res. 2012a;140(1-3):262–263. doi: 10.1016/j.schres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr Q. 2012b;83(1):91–102. doi: 10.1007/s11126-011-9186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinkova L, Tuckova L, Cinova J, Flegelova Z, Tlaskalova-Hogenova H. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004;571(1-3):81–85. doi: 10.1016/j.febslet.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Morden NE, Austin K, Ilgen M, McCarthy JF, Dalack G, Blow FC. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31(6):555–563. doi: 10.1016/j.genhosppsych.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135(1):194–204. e193. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol. 2012;26(5 Suppl):33–41. doi: 10.1177/0269881111431622. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29(1):117–124. [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand. 2013;127(5):344–354. doi: 10.1111/j.1600-0447.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Mellor A, Buckley P. Total and differential white blood cell counts, highsensitivity C-reactive protein, and the metabolic syndrome in non-affective psychoses. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12(4):186–192. doi: 10.1016/j.tim.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Muller N, Myint AM, Schwarz MJ. Inflammation in schizophrenia. Adv Protein Chem Struct Biol. 2012a;88:49–68. doi: 10.1016/B978-0-12-398314-5.00003-9. [DOI] [PubMed] [Google Scholar]
- Muller N, Wagner JK, Krause D, Weidinger E, Wildenauer A, Obermeier M, Dehning S, Gruber R, Schwarz MJ. Impaired monocyte activation in schizophrenia. Psychiatry Res. 2012b;198(3):341–346. doi: 10.1016/j.psychres.2011.12.049. [DOI] [PubMed] [Google Scholar]
- Niebuhr DW, Li Y, Cowan DN, Weber NS, Fisher JA, Ford GM, Yolken R. Association between bovine casein antibody and new onset schizophrenia among US military personnel. Schizophr Res. 2011;128(1-3):51–55. doi: 10.1016/j.schres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Van Baal GC, Vonk R, Wijkhuijs AJ, Kahn RS, Nolen WA, Drexhage HA. Genetic and environmental influences on pro-inflammatory monocytes in bipolar disorder: a twin study. Arch Gen Psychiatry. 2009;66(9):957–965. doi: 10.1001/archgenpsychiatry.2009.116. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Knivsberg AM. The possibility and probability of a gut-to-brain connection in autism. Ann Clin Psychiatry. 2009;21(4):205–211. [PubMed] [Google Scholar]
- Romero-Sanchez M, Gonzalez-Serna A, Pacheco YM, Ferrando-Martinez S, Machmach K, Garcia-Garcia M, Alvarez-Rios AI, Vidal F, Leal M, Ruiz-Mateos E. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65(5):431–438. doi: 10.1016/j.jinf.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Samaroo D, Dickerson F, Kasarda DD, Green PH, Briani C, Yolken RH, Alaedini A. Novel immune response to gluten in individuals with schizophrenia. Schizophr Res. 2010;118(1-3):248–255. doi: 10.1016/j.schres.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012a;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Halling M, Krivogorsky B, Haile L, Yang S, Stallings CR, Origoni AE, Bossis I, Xiao J, Dupont D, Haasnoot W, Yolken RH. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr Res. 2010a;118(1-3):240–247. doi: 10.1016/j.schres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Severance EG, Dupont D, Dickerson FB, Stallings CR, Origoni AE, Krivogorsky B, Yang S, Haasnoot W, Yolken RH. Immune activation by casein dietary antigens in bipolar disorder. Bipolar Disord. 2010b;12(8):834–842. doi: 10.1111/j.1399-5618.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Alaedini A, Dupont D, Dickerson FB, Yolken RH. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis. 2012b;48(3):447–453. doi: 10.1016/j.nbd.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Kannan G, Gressitt KL, Xiao J, Alaedini A, Pletnikov MV, Yolken RH. Anti-gluten immune response following Toxoplasma gondii infection in mice. PLoS One. 2012c;7(11):e50991. doi: 10.1371/journal.pone.0050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares FL, de Oliveira Matoso R, Teixeira LG, Menezes Z, Pereira SS, Alves AC, Batista NV, de Faria AM, Cara DC, Ferreira AV, Alvarez-Leite JI. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2012.08.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stehle JR, Jr., Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67(11):1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Schiltz K, Muller UJ, Westphal S, Drexhage HA, Bogerts B. Immune system and glucose metabolism interaction in schizophrenia: A chicken-egg dilemma. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.09.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clement K, Hu FB, Lin X. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33(9):1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvisaari J, Partti K, Perala J, Viertio S, Saarni SE, Lonnqvist J, Saarni SI, Harkanen T. Mortality and its determinants in people with psychotic disorder. Psychosom Med. 2013;75(1):60–67. doi: 10.1097/PSY.0b013e31827ad512. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176(4):2512–2521. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Tuckova L, Stepankova R, Hudcovic T, Palova-Jelinkova L, Kozakova H, Rossmann P, Sanchez D, Cinova J, Hrncir T, Kverka M, Frolova L, Uhlig H, Powrie F, Bland P. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann N Y Acad Sci. 2005;1051:787–798. doi: 10.1196/annals.1361.122. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, Seim A, Pedersen L, Schondel M, Shattock P. The ScanBrit randomised, controlled, singleblind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci. 2010;13(2):87–100. doi: 10.1179/147683010X12611460763922. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Shattock P, Knivsberg AM, Seim A, Reichelt KL, Todd L, Carr K, Hooper M. Gluten- and casein-free dietary intervention for autism spectrum conditions. Front Hum Neurosci. 2012;6:344. doi: 10.3389/fnhum.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]