Stable incidence but increase in prevalence of ANCA-associated vasculitis in southern Sweden: a 23-year study (original) (raw)
Abstract
Objective
To update the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) in a defined geographical area of southern Sweden.
Methods
The study area comprised 14 municipalities with a combined adult population (≥18 years) of 623 872 in 2019. All cases diagnosed with AAV in 1997–2019 in the study area were included in the estimate of incidence. Diagnosis of AAV was verified by case record review, and cases were classified using the European Medicines Agency algorithm. Point prevalence was estimated on 01 January 2020.
Results
Three hundred and seventy-four patients (median age 67.5 years, 47% female) were diagnosed with new-onset AAV during the study period. One hundred and ninety-two were classified as granulomatosis with polyangiitis (GPA), 159 as microscopic polyangiitis (MPA) and 23 as EGPA. The average annual incidence/million adults was 30.1 (95% CI 27.0 to 33.1) for AAV: 15.4 (95% CI 13.3 to 17.6) for GPA, 12.8 (95% CI 10.8 to 14.8) for MPA and 1.8 (95% CI 1.1 to 2.6) for eosinophilic GPA (EGPA). Incidence was stable during the study period, 30.3/million 1997–2003, 30.4/million 2004–2011 and 29.5/million 2012–2019. The incidence increased with age and was highest in age group 70–84 years (96/million adults). On 1 January 2020, the prevalence was 428/million adults and was higher in males than in females (480 vs 378/million).
Conclusions
The incidence of AAV in southern Sweden was found stable over the course of 23 years; while the prevalence has increased, which might indicate better management and treatment of AAV resulting in improved survival.
Keywords: Systemic vasculitis, Epidemiology, Granulomatosis with polyangiitis
WHAT IS ALREADY KNOWN ON THIS TOPIC
- Increasing incidence and prevalence of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis have been described in earlier studies.
WHAT THIS STUDY ADDS
- Observation of stable incidence (30 cases per million) and rising prevalence (highest ever reported prevalence with 428 cases per million) in a population-based cohort with 23-year follow-up time.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
- The incidence of ANCA-associated vasculitis (AAV) is stable during a long observation period in this large population-based cohort from Sweden, implying that changes in disease definitions and classification criteria as well as increased physician awareness and ANCA testing might explain rising incidence in earlier studies rather than true incidence increases. The prevalence is the highest ever reported in AAV, indicating improved management and, therefore, survival.
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) comprises a group of rare diseases that are classified according to clinicopathological characteristics into three phenotypic variants: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA).1 The disease primarily affects small blood vessels, leading to a range of pathological changes including granulomatous or necrotising inflammation in the affected organ systems. It commonly affects multiple organs, primarily lungs, kidneys and skin as well as the upper respiratory tract and the sinonasal area. Anti-neutrophil cytoplasmic antibodies, predominantly of IgG type, targeting myeloperoxidase (MPO) or proteinase-3 (PR3) can frequently be detected.2 AAV is associated with disease-related and/or treatment-related morbidity and mortality.3 4 Epidemiological studies of rare diseases such as AAV are needed to increase our understanding of aetiological mechanisms and provide a basis for the planning and allocation of healthcare resources. Common challenges in studying the epidemiology of rare diseases are differences in methods of case identification and ascertainment, small sample sizes and low incidence.5 Development and revision of disease definitions and classification criteria have substantially improved the comparability of findings of epidemiological studies in AAV in recent decades.1 6 7 Today ANCA testing is routine in diagnostic workup of AAV.8 MPO-AAV and PR3-AAV differ genetically9 10 and in disease course,11 and discussion of a shift from classification based on phenotype to one based on serotype is ongoing.12 13
The reported incidence of AAV has increased globally in recent decades.14 From 1998 to 2015, studies in European countries reported annual incidence of all AAV phenotypes per million inhabitants of 10.2 in northern Germany (1998–1999),15 12.2 (1998–2001) in north-western Spain,16 18.5 (2000–2015) in Denmark,17 20.4 in the UK (1988–1997),18 20.8 (1997–2006)19 in Sweden and 24.7 (99–2013)20 in Norway. Comparable incidences have been reported in Japan but with significant differences in disease phenotype and serotype distribution. Most patients were classified as MPA, with the dominating serology type being MPO-ANCA.21 22 The increasing incidence might be partly explained by changes in classification criteria along with increased physician awareness and availability of ANCA testing. However, in addition to geographical and demographic differences among the cited studies, methods of case identification/ascertainment and classification criteria differed, so variation and increases in incidence might be explained by factors other than true changes in the epidemiology of the disease.
Studies have reported incidences of AAV to peak in winter,23 summer24 or to show no seasonal variation.25
An increase in incidence, improved case identification and greater survival rates are all possible factors in rising prevalence of AAV, as reported in several studies. In the UK and northern Germany, the reported prevalence of GPA and MPA has doubled from the 1990s to 2006.26 Prevalence of AAV was reported as 181/million in Tromsö, Norway in 2003 compared with 352 in 2013.20 More recently, a prevalence rate of 421/million from Olmsted County in the USA was reported.27
Due to changing incidence and prevalence around the world, we present an update comparing incidence and prevalence of AAV over a 23-year period using the same case definition and classification as in our previous study.19 We also aim to assess possible seasonal variation in disease onset.
Methods
The study area
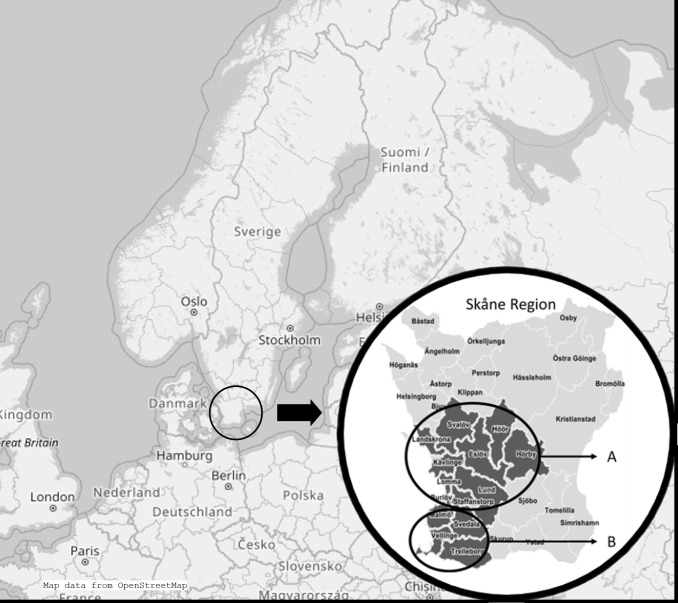
The study area comprised 14 municipalities in southern Sweden. The study area includes both urban and rural areas, with a mean population density of 333/km2 compared with 25/km2 for the country as a whole. Most people live in cities. The previously described study area19 28 is served by four hospitals. Skåne university hospital, with campuses in Lund and Malmö, is a tertiary university hospital and referral centre for nearly 1.7 million people. Hospitals in the cities of Landskrona and Trelleborg also serve the area, both with internal medicine in-patient services. Study areas designated A and B were used for the estimates of incidence and comorbidities, and area A was used for prevalence estimates (figure 1).
Figure 1.
Study area A and B within the Skåne region in the southernmost part of Sweden.
The population
The total adult population (≥18 years) was 623 872 (51% female) in December 2019. The adult population in the study area increased by 140 574 (29%) from 1997 to 2019. The age distribution in the study area was 40%, 18–39 years; 25%, 40–54; 20%, 55–69, 12%, 70–84 and 3%, >85 and was stable throughout study time.
The population structure of Sweden has undergone changes between 2000 and 2019. Specifically, the percentage of immigrants born in Asia and Africa has increased threefold. The percentage of people from Africa increased from 0.6% to 2.2%, while the percentage of people from Asia increased from 2.5% to 7.6%. In contrast, the percentage of people from other Nordic countries and the EU remained constant at around 5.5%. Immigration from non-EU Europe, Oceania and the Americas is also similar. Most immigrants are between the ages of 25 and 40.29
Registries and databases
The Skåne Healthcare Register (SHR) is a comprehensive administrative register containing information regarding healthcare services in the region of Skåne, Sweden, since 1998.30 All healthcare contacts including visits to physicians, nurses and other healthcare professionals, along with a record of care provided, are registered under the patient’s unique 10-digit personal identity number. Diagnoses are reported in the SHR using the International Classification of Diseases, 10th revision (ICD-10). The SHR was also used to identify comorbidities in the prevalent cases.
The ANCA-serology databases at the department of clinical immunology in Lund and the private laboratory SVAR (formerly known as Wieslab AB) were used to identify patients testing positive for PR3-ANCA and MPO-ANCA.
The population registry was used to access the residential address of cases. Only cases diagnosed while living in the study area were included as incident cases. For the prevalence estimates, only patients living in the study area at date of prevalence estimate were included.
Case retrieval
Case retrieval was slightly modified during the study period. In the first 6 years of the study,28 three retrieval sources (ANCA-serology, clinical and histology registries) were accessed. In our seminal study of the prevalence of primary systemic vasculitis including AAV, 80 of 86 (93%) cases were identified by combining the diagnosis registries at the Department of Nephrology and the Department of Rheumatology at Skåne University Hospital with the ANCA database.28 After 2003, only the diagnosis registry using the ICD-10 codes (M30.1–M31.9) and the ANCA database were used for case identification. The SHR entries for ICD10-codes M30.1–M31.9 were retrieved in 3 year increments.30 Similarly, the serology databases were periodically searched for persons with positive tests for either PR3-ANCA or MPO-ANCA at the two laboratories analysing ANCA serology in the study area.
Case ascertainment and classification
Records of all potentially eligible cases identified from the retrieval sources were reviewed to confirm a diagnosis of small vessel vasculitis as defined by the European Medicines Agency (EMA) algorithm,7 which was also employed for disease classification. The diagnosis of small vessel vasculitis was based on symptoms compatible with, or typical of, vasculitis and supported by histological or serological findings. If biopsy was not conclusive or not performed, surrogate markers for small vessel vasculitis or granulomatous disease were used as previously described.7 It is also required that no other diagnosis could explain the clinical presentation. Cases fulfilling criteria were classified into AAV-phenotypes employing the EMA algorithm.7
Data collection
Demographics and clinical data, including organ involvement, along with routine laboratory, serology, imaging and histology data, were collected for cases at time of diagnosis and during follow-up when available. Information of selected comorbidities (myocardial infarction, stroke, cancer, hypertension and diabetes mellitus) occurring after diagnosis of AAV was collected by linking the AAV cohort to the SHR using the corresponding ICD-10 codes for these diseases. Place of residence and vital status were acquired from the Swedish population register. End-stage renal disease (ESRD) was defined as ongoing dialysis or successful renal transplant. Information with respect to ESRD was collected from case records.
Statistical analysis
Data are presented as frequency and percentage, mean and SD, or median with IQR when appropriate. Categorical variables were compared by using χ2 test. Data of study population used for the calculation of incidence and point prevalence (PP) were acquired from Statistics Sweden.29 For incidence estimates, all individuals newly diagnosed with AAV from 1997 to 2019 living within the study area at the time of diagnosis were included. The incidence per million adults for each calendar year during the study period was calculated using the total adult population each year on 1 January as the denominator and number of patients diagnosed in that year as the numerator. Incidences were estimated in 3-year overlapping periods (1997–1999, 1998–2000, 1999–2001, etc) to illustrate changes in incidence rate overtime. For calculation of age-specific incidence, the cohort was separated into age groups: 18–39, 40–54, 55–69, 70–84 and ≥85 years.
For study of seasonal variation, winter was defined as December–February; spring, March–May; summer, June–August; and autumn, September–November. A Poisson regression model was used to estimate seasonal incidence ratios, with winter as reference.
We estimated the prevalence at four time points, 2003, 2010, 2015 and 2020, with the pp per million adults estimated on 1 January 2020 as a measure of overall prevalence. To allow follow-up and comparison of prevalence figures in southern Sweden over time, we selected pp data only from study area A, which was the site of our first report in 200728 (figure 1). Data from the earlier study were recalculated to reflect only prevalence in the adult population.28 The denominator of prevalence estimate was adult population at pp dates. The numerator was the number of prevalent cases living in study area A on the corresponding date. A normal approximation method was used for the calculation of CIs for incidence and prevalence estimates. To give a relative measure of disease burden on the pp date, selected comorbidities diagnosed after the onset of AAV were identified and presented as number and percentage. All statistical calculations were conducted with Statistical Package for the Social Sciences, SPSS V.26 for Windows (IBM SPSS Statistics).
Results
Patients
Three hundred and seventy-four cases (47% female) were diagnosed with AAV during the 23-year study period. The median age at diagnosis was 67.5 (IQR 55–77) years for all patients, 68 (IQR 56.2–77) for males and 67 (IQR 53–77.2) for females. One hundred and ninety-two patients were classified as GPA, 159 as MPA and 23 as EGPA. One hundred and eighty-eight were PR3-ANCA positive, 161 were MPO-ANCA positive and 25 patients were ANCA negative. Patients diagnosed with MPA were older at time of diagnosis (median age 72 (IQR 60–81)) than those with GPA (median age 64 (IQR 52–73)) and EGPA (median age 60 (IQR 37–63)).
Incidence
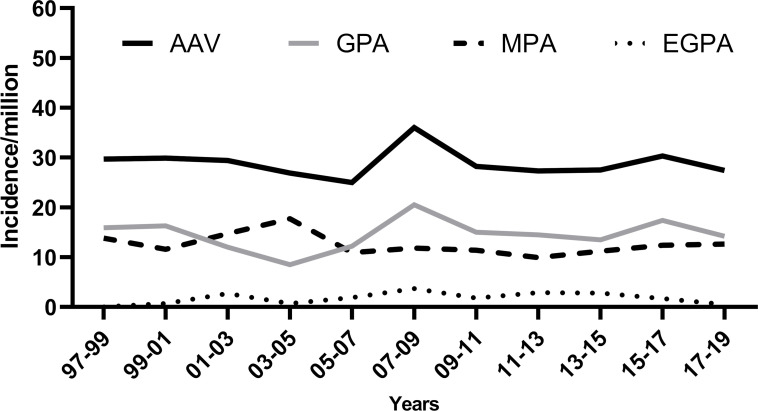
The calculated mean annual incidence 1997–2019 for AAV was 30.1 per million adults (95% CI 27.0 to 33.1) for all AAV, 15.4 (95%CI 13.3 to 17.6) for GPA, 12.8 (95%CI 10.8 to 14.8) for MPA and 1.8 (95%CI 1.1 to 2.6) for EGPA. The incidence of AAV was higher in males, 32.9 (95% CI 28.4 to 37.5) than in females 27.3 (95% CI 23.3 to 31.4) (table 1). The mean annual incidence from 1997 to 2003 was 30.3 per million (95% CI 24.4 to 36.1), 30.4 per million (95% CI 25.2 to 35.7) 2004–2011 and 29.5 per million (95% CI 24.6 to 34.4) from 2012 to 2019. The incidence was estimated in 3-year overlapping periods and was relatively stable throughout the study period (figure 2).
Table 1.
Average annual incidence per million adults for AAV based on disease phenotype and ANCA serology
| AAVn=374(95% CI) | GPAn=192(95% CI) | MPAn=159(95% CI) | EGPAn=23(95% CI) | PR3 +n=188(95% CI) | MPO +n=161(95% CI) | |
|---|---|---|---|---|---|---|
| All | 30.1(27.0 to 33.1) | 15.4(13.3 to 17.6) | 12.8(10.8 to 14.8) | 1.8(1.1 to 2.6) | 15.0(12.9 to 17.2) | 12.9(10.9 to 14.9) |
| Male | 32.9(28.4 to 37.5) | 18.3(14.9 to 21.7) | 13.2(10.3 to 16.1) | 1.5(0.5 to 2.4) | 18.8(15.3 to 22.2) | 12.2(9.4 to 15.0) |
| Female | 27.3(23.3 to 31.4) | 12.7(10.0 to 15.5) | 12.4(9.7 to 15.1) | 2.2(1.0 to 3.4) | 11.5(8.8 to 14.1) | 13.7(10.8 to 16.5) |
Figure 2.
Incidence of AAV per million adults, stratified by 3 years overlapping, for all AAV and different phenotypes. AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Age-specific and sex-specific incidence
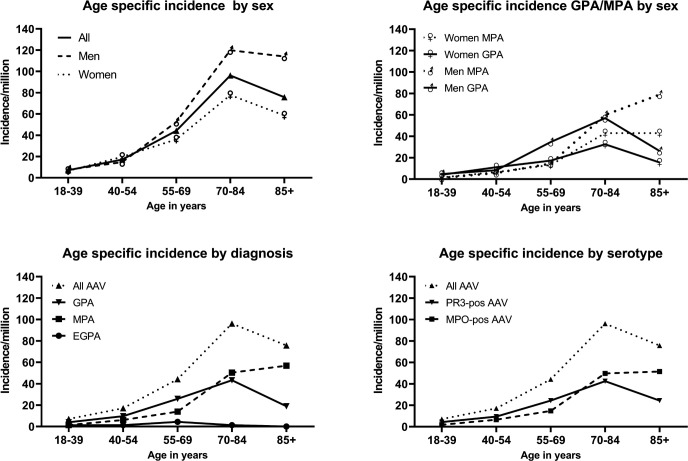
The incidence of AAV increased with age. The highest incidence was in the age group 70–84 years: 96.2 per million adults (95% CI 80.6 to 111.7) overall, 77.8 per million (95% CI 59.2 to 96.4) females and 119.9 per million (95% CI 93.6 to 146.0) males (figure 3). Highest GPA incidence rates were also observed in those 70–84 years old (43.2 per million) and decreased in the age group ≥85 years to 18.9 per million. The peak incidence of MPA was observed in the age group ≥85 years at 56.8 per million. This age-related increase of MPA incidence could be attributed to males, in whom we observed an increase from 59.9 per million (70–84 years) to 78.9 per million (≥85). In females, incidence was similar in these age groups (43 per million). Highest incidence of EGPA was observed in those 55–69 years (4.4 per million) (figure 3). Age-specific incidence by serotype was nearly identical to phenotype distribution (figure 3).
Figure 3.
Age specific incidence rates in respect to phenotype, serology and sex. AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Seasonal variation
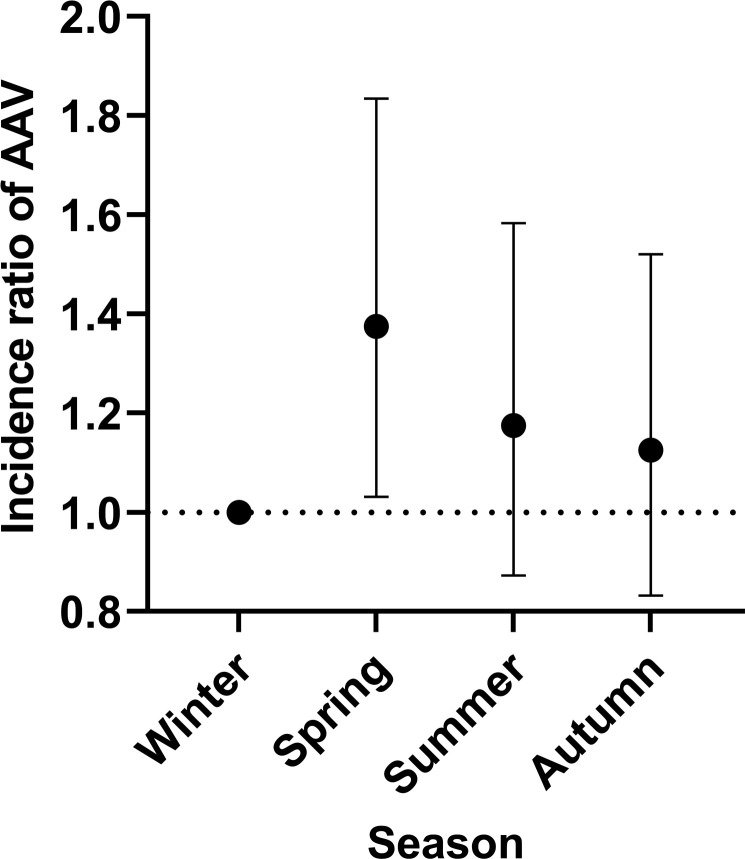
The seasonal distribution of AAV diagnosis was 80 cases (21%) in winter, 110 (29%) in spring, 94 (25%) in summer and 90 (24%) in autumn. The greatest number of cases were diagnosed in March and May (both n=37), followed by April (n=36). With winter as reference, a significantly higher incidence ratio (IR) was observed in spring (IR 1.38 (95% CI 1.03 to 1.83)) for all AAV (figure 4), 1.43 (95% CI 0.90 to 2.14) for GPA and 1.45 (95% CI 0.90 to 2.26) for MPA. EGPA showed higher numbers in winter. The incidence ratios for spring with winter as a reference were 1.27 (95% CI 0.80 to 1.91) for PR3-positive disease and 1.59 (95% CI 1.03 to 2.48) for MPO-positive disease.
Figure 4.
Seasonal variations. Incidence ratios with 95% CI of AAV-diagnoses in seasons (winter is reference). AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis.
Point prevalence
Data of prevalence prior to the study period (1996 and earlier) were only available for 10 of the 14 municipalities in the study area with a total adult population of 273 135 (designated study area A). In area A, 50 patients were diagnosed with AAV prior to 1997. Twenty-five of these were still living in the study area at pp 1 January 2020, along with a further 92 incident cases, resulting in 117 patients included in 2020 prevalence estimates. The prevalence of AAV on 1 January 2020 was 428.4 per million adults (95% CI 350.7 to 506.0) for all patients (table 2), 241.6 (95% CI 183.3 to 299.9) for GPA, 150.1 (95% CI 104.2 to 196.1) for MPA and 36.6 (95% CI 13.9 to 59.3) for EGPA. The median age at pp 2020 was 71.5 (IQR 60.1–78.2) for all patients, 71.1 (IQR 56.1–75.7) for GPA, 74.2 (IQR 65.1–81.8) for MPA and 65.5 (IQR 52.1–79.5) for EGPA. The sex-specific prevalence was 377.8 (95% CI 275.1 to 480.5) in women and 479.7 (95% CI 363.1 to 596.4) in men. In comparison, prevalence for all AAV in 2003 was recalculated using the adult population as denominator and was 353.6 per million adults (95% CI 275.6 to 431.6). The prevalence at four time points is shown in table 2.
Table 2.
Point prevalence (PP) of ANCA-associated vasculitis (AAV) per million adults
| N | AAV pp(95% CI) | n | GPA pp(95% CI) | n | MPA pp(95% CI) | n | EGPA pp(95% CI) | |
|---|---|---|---|---|---|---|---|---|
| 2003 | 79 | 353.6(275.6 to 431.6) | 49 | 219.3(157.9 to 280.7) | 24 | 107.4(64.4 to 150.4) | 6 | 26.9(5.4 to 48.32) |
| 2010 | 107 | 434.7(352.3 to 517.1) | 58 | 235.6(175.0 to 296.3) | 40 | 162.5(112.1 to 212.9) | 9 | 36.6(12.7 to 60.5) |
| 2015 | 121 | 468.9(385.3 to 552.4) | 68 | 263.5(200.9 to 326.1) | 42 | 162.8(113.5 to 212.0) | 11 | 42.6(17.4 to 67.8) |
| 2020 | 117 | 428.4(350.7 to 506.0) | 66 | 241.6(183.3 to 299.9) | 41 | 150.1(104.2 to 196.1) | 10 | 36.6(13.9 to 59.3) |
Comorbidities
On 01 January 2020, 220 patients with AAV were living in the study area. The most common comorbidity was hypertension (50%, n=110), followed by cancer (27%, n=59) and diabetes (19.5%, n=43). At pp 2020, 135 patients (61%) had suffered at least one comorbidity and 86 (39%) had suffered two comorbidities. Online supplemental table S1 lists comorbidities diagnosed in prevalent cases following diagnosis of AAV.
Supplementary data
rmdopen-2022-002949supp002.pdf (22.4KB, pdf)
Discussion
We report the incidence and prevalence of AAV in southern Sweden using a well-defined population-based cohort diagnosed over a period of 23 years. Incidence of AAV was relatively stable throughout the study period. Incidence increased with age and showed seasonal variation, with a higher number of patients diagnosed during spring. We observed an increase in prevalence during the study period with, to the best of our knowledge, highest prevalence ever reported for AAV.
We used similar retrieval sources, case identification and classification system throughout the 23-year study period, eliminating other variables that might explain the observed stability of incidence. A previous Swedish study relying on ICD-10 codes in a national registry demonstrated progressive increase in incidence of GPA from the early 1970s–2001.31 Watts et al18 similarly describe an increase in a 10-year study in Norwich, UK, as do Takala et al32 in Finland. A study from Taiwan33 also reports rising incidence. The most likely source of this apparent increase in incidence is the introduction of ANCA testing in the mid-1980s and early 1990s,34 the publication of ACR 1990 classification criteria of GPA and EGPA,6 35 and the CHCC 1994 definitions of vasculitis.36 Our results suggest that AAV incidence may have been stable during this time period and the observed differences may be attributed to changes in diagnosis and classification of AAV rather than a genuine increase in the incidence. Our results are in agreement with the most recently reported studies from Norway20 and the USA.27
Earlier findings of latitudinal differences, the so called ‘north-south gradient’ in the relative incidence of GPA and MPA37 are not evident in this cohort, as incidences of GPA and MPA are similar, which is in line with our earlier findings.19 The incidence of EGPA is comparable to reported data from the UK, 2.7 per million (95% CI 1.3 to 4.8),18 Australia, 2.2 per million (95% CI 0.6 to 7.2)38 and the USA, 4 per million (95% CI 1.0 to 6.0).27
The incidence of AAV increases with age. Peak incidence was at 70–84 years. The peak age at disease onset differs with disease phenotype, and was 70–84 years for GPA, while incidence of MPA peaks in the ≥85 years age group. The peak age at onset was greater than reported in previous studies of primary systemic vasculitis and GPA from the UK,18 Finland32 and Norway,39 where incidence peaked in the age group 65–74 years and in Spain where it was 55–64 years.16 Pearce et al reported a peak age at diagnosis of≥85 years40 in a study from the UK including patients with AAV diagnosed from 2007 to 2013. When we reanalysed our cohort including only patients diagnosed 2007–2019, we observed similar findings, with highest incidence in the age group ≥85 years (online supplemental figure S2). This finding might indicate a shift towards older age at diagnosis possibly related to increased physician awareness and the widespread availability and use of ANCA testing, regardless of age or comorbidity burden.
Supplementary data
rmdopen-2022-002949supp001.pdf (301KB, pdf)
We observed highest incidence of AAV diagnosis in spring. Several studies have discussed seasonal variation in incidence of AAV, but findings are contradictory. Falk et al41 reported highest incidence in winter for all AAV. A French study of GPA found highest incidence in summer,42 others found no seasonal variation.43 Differences in study design, case definition and data collection might explain these differences and limit comparability.
Studying seasonality at diagnosis of AAV aims to shed light on etiological aspects of allergic or infectious triggers.42 44 The result of seasonal analysis in this study supports the recently published data from our group demonstrating increased risk of AAV following infections, especially those affecting the respiratory tract.44 A plausible pathophysiologic mechanism is that respiratory infection during winter may trigger the onset of AAV a few months later. A recent study from Scotland of GPA and MPA did not show seasonality,25 but comparability is limited because of differences in study population. Patients in the study were recruited via a renal biopsy register, and the fraction of MPA patients was much higher, not representative of the entire spectrum of AAV as is our population-based cohort.
The prevalence of AAV in our area increased from 2003 to 2015 and slightly decreased after 2015. The increase in prevalence despite stable incidence might be attributed to improvements in patient survival, physician awareness and treatment. During the study period, there was a shift to less toxic treatment regimens,45 as well the use of biological therapy. Studies of PP aim to assess burden of disease in a population to scale the dimension of future healthcare resources needed. In addition to the challenges that increased AAV prevalence implies, these patients are more prone to comorbidities.46
Strengths of this study include the population-based setting, with no referral or selection bias, and the inclusion of only patients with confirmed clinical diagnosis of small vessel vasculitis. In addition, all were classified using the same algorithm over a long period of time. This study includes a large population-based cohort encompassing the entire spectrum of AAV from mild disease to severe multisystemic vasculitis with continuous inclusion of patients using similar case identification criteria. A further strength is a validated search strategy with high case identification and completeness and robust case ascertainment. The long study period enabled us to assess incidence over time as well as seasonal variation of the disease.
The incidence of AAV in southern Sweden was found stable over the course of 23 years; while the prevalence has increased, which might indicate better management and treatment of AAV resulting in improved survival.
Footnotes
Contributors: All authors were involved in drafting the article or revising it for intellectual content, and all authors approved the final version to be published. Study conception and design: all authors. Data acquisition and analysis: JR, AJM and MS. Statistical analysis: JR. Interpretation of data: all authors. Guarantor: AJM.
Funding: This study was supported by grants to AJM from the Swedish Research Council (Vetenskapsrådet: 2019-01655); Faculty of Medicine, Lund University (ALF-medel); Alfred Österlund’s Foundation; King Gustaf V's 80-year foundation; Swedish Rheumatology Association (Reumatikerförbundet); Anna Greta Crafoord’s Foundation.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was performed in accordance with Declaration of Helsinki and was approved by the regional ethical review board in Lund, Sweden (2010/517). Informed consent was not acquired according to ethical approval. An informed consent was not required by the ethical board.
References
- 1.Jennette JC, Falk RJ, Bacon PA. Overview of the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603–6. 10.1007/s10157-013-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA). Autoimmunity 2005;38:93–103. 10.1080/08916930400022673 [DOI] [PubMed] [Google Scholar]
- 3.Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. 10.1136/ard.2009.109389 [DOI] [PubMed] [Google Scholar]
- 4.Flossmann O, Berden A, de Groot K, et al. Long-Term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. 10.1136/ard.2010.137778 [DOI] [PubMed] [Google Scholar]
- 5.Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford) 2020;59:iii42–50. 10.1093/rheumatology/keaa089 [DOI] [PubMed] [Google Scholar]
- 6.Hunder GG, Arend WP, Bloch DA, et al. The American College of rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum 1990;33:1065–7. 10.1002/art.1780330802 [DOI] [PubMed] [Google Scholar]
- 7.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. 10.1136/ard.2006.054593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossuyt X, Cohen Tervaert J-W, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683–92. 10.1038/nrrheum.2017.140 [DOI] [PubMed] [Google Scholar]
- 9.Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367:214–23. 10.1056/NEJMoa1108735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons PA, Peters JE, Alberici F, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun 2019;10:5120. 10.1038/s41467-019-12515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammad AJ, Segelmark M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA) -associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 2014;41:1366–73. 10.3899/jrheum.131038 [DOI] [PubMed] [Google Scholar]
- 12.Hilhorst M, van Paassen P, Tervaert JWC, et al. Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol 2015;26:2314–27. 10.1681/ASN.2014090903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windpessl M, Bettac EL, Gauckler P, et al. ANCA status or clinical phenotype- what counts more? Curr Rheumatol Rep 2021;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watts RA, Hatemi G, Burns JC, et al. Global epidemiology of vasculitis. Nat Rev Rheumatol 2022;18:22–34. 10.1038/s41584-021-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, et al. No difference in the incidences of vasculitides between North and South Germany: first results of the German vasculitis register. Rheumatology (Oxford) 2002;41:540–9. 10.1093/rheumatology/41.5.540 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, et al. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the chapel Hill consensus conference definitions. Arthritis Rheum 2003;49:388–93. 10.1002/art.11115 [DOI] [PubMed] [Google Scholar]
- 17.Nelveg-Kristensen KE, Szpirt W, Carlson N, et al. Increasing incidence and improved survival in ANCA-associated vasculitis-a Danish nationwide study. Nephrol Dial Transplant 2021;37:63–71. 10.1093/ndt/gfaa303 [DOI] [PubMed] [Google Scholar]
- 18.Watts RA, Lane SE, Bentham G, et al. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000;43:414–9. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad AJ, Jacobsson LTH, Westman KWA, et al. Incidence and survival rates in Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology (Oxford) 2009;48:1560–5. 10.1093/rheumatology/kep304 [DOI] [PubMed] [Google Scholar]
- 20.Nilsen AT, Karlsen C, Bakland G, et al. Increasing incidence and prevalence of ANCA-associated vasculitis in northern Norway. Rheumatology (Oxford) 2020;59:2316–24. 10.1093/rheumatology/kez597 [DOI] [PubMed] [Google Scholar]
- 21.Watts RA, Scott DGI, Jayne DRW, et al. Renal vasculitis in Japan and the UK -- are there differences in epidemiology and clinical phenotype? Nephrol Dial Transplant 2008;23:3928–31. 10.1093/ndt/gfn354 [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto S, Watts RA, Kobayashi S, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology (Oxford) 2011;50:1916–20. 10.1093/rheumatology/ker205 [DOI] [PubMed] [Google Scholar]
- 23.Draibe J, Rodó X, Fulladosa X, et al. Seasonal variations in the onset of positive and negative renal ANCA-associated vasculitis in Spain. Clin Kidney J 2018;11:468–73. 10.1093/ckj/sfx127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahr A, Artigues N, Coste J, et al. Seasonal variations in onset of wegener’s granulomatosis: increased in summer? J Rheumatol 2006;33:1615–22. [PubMed] [Google Scholar]
- 25.Aiyegbusi O, Frleta-Gilchrist M, Traynor JP, et al. Anca-Associated renal vasculitis is associated with rurality but not seasonality or deprivation in a complete national cohort study. RMD Open 2021;7:e001555. 10.1136/rmdopen-2020-001555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts RA, Mahr A, Mohammad AJ, et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA) -associated vasculitis. Nephrol Dial Transplant 2015;30 Suppl 1:i14–22. 10.1093/ndt/gfv022 [DOI] [PubMed] [Google Scholar]
- 27.Berti A, Cornec D, Crowson CS, et al. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in Olmsted County, Minnesota: a twenty-year us population-based study. Arthritis Rheumatol 2017;69:2338–50. 10.1002/art.40313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad AJ, Jacobsson LTH, Mahr AD, et al. Prevalence of Wegener’s granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology (Oxford) 2007;46:1329–37. 10.1093/rheumatology/kem107 [DOI] [PubMed] [Google Scholar]
- 29.Folkmängd i riket, län och kommuner 31 december 2020 och befolkningsförändringar 2020. Statistics Sweden, 2020. Available: http://www.scb.se [Google Scholar]
- 30.Löfvendahl S, Schelin MEC, Jöud A. The value of the skåne health-care register: prospectively collected individual-level data for population-based studies. Scand J Public Health 2020;48:56–63. 10.1177/1403494819868042 [DOI] [PubMed] [Google Scholar]
- 31.Knight A, Ekbom A, Brandt L, et al. Increasing incidence of wegener’s granulomatosis in sweden, 1975-2001. J Rheumatol 2006;33:2060–3. [PubMed] [Google Scholar]
- 32.Takala JH, Kautiainen H, Malmberg H, et al. Incidence of wegener’s granulomatosis in finland 1981-2000. Clin Exp Rheumatol 2008;26:S81–5. 10.1080/03009740802238366 [DOI] [PubMed] [Google Scholar]
- 33.Wu C-S, Hsieh C-J, Peng Y-S, et al. Antineutrophil cytoplasmic antibody-associated vasculitis in taiwan: a hospital-based study with reference to the population-based national health insurance database. J Microbiol Immunol Infect 2015;48:477–82. 10.1016/j.jmii.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 34.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet 1985;1:425–9. 10.1016/s0140-6736(85)91147-x [DOI] [PubMed] [Google Scholar]
- 35.Masi AT, Hunder GG, Lie JT, et al. The American College of rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–100. 10.1002/art.1780330806 [DOI] [PubMed] [Google Scholar]
- 36.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Arthritis & Rheumatism 1994;37:187–92. 10.1002/art.1780370206 Available: http://doi.wiley.com/10.1002/art.v37:2 [DOI] [PubMed] [Google Scholar]
- 37.Watts RA, Lane SE, Scott DG, et al. Epidemiology of vasculitis in Europe. Ann Rheum Dis 2001;60:1156–7. 10.1136/ard.60.12.1156a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ormerod AS, Cook MC. Epidemiology of primary systemic vasculitis in the Australian Capital Territory and south-eastern New South Wales. Intern Med J 2008;38:816–23. 10.1111/j.1445-5994.2008.01672.x [DOI] [PubMed] [Google Scholar]
- 39.Koldingsnes W, Nossent H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheum 2000;43:2481–7. [DOI] [PubMed] [Google Scholar]
- 40.Pearce FA, Lanyon PC, Grainge MJ, et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology (Oxford) 2016;55:1656–63. 10.1093/rheumatology/kew232 [DOI] [PubMed] [Google Scholar]
- 41.Falk RJ, Hogan S, Carey TS, et al. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The glomerular disease collaborative network. Ann Intern Med 1990;113:656–63. 10.7326/0003-4819-113-9-656 [DOI] [PubMed] [Google Scholar]
- 42.Mahr A, Artigues N, Coste J, et al. Seasonal variations in onset of Wegener’s granulomatosis: increased in summer? J Rheumatol 2006;33:1615–22. [PubMed] [Google Scholar]
- 43.Aries PM, Herlyn K, Reinhold-Keller E, et al. No seasonal variation in the onset of symptoms of 445 patients with Wegener’s granulomatosis. Arthritis Rheum 2008;59:904. 10.1002/art.23722 [DOI] [PubMed] [Google Scholar]
- 44.Rathmann J, Stamatis P, Jönsson G, et al. Infection is associated with increased risk of MPO- but not PR3-ANCA-associated vasculitis. Rheumatology (Oxford) 2022;61:4817–26. 10.1093/rheumatology/keac163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 46.Englund M, Merkel PA, Tomasson G, et al. Comorbidities in patients with antineutrophil cytoplasmic antibody-associated vasculitis versus the general population. J Rheumatol 2016;43:1553–8. 10.3899/jrheum.151151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
rmdopen-2022-002949supp002.pdf (22.4KB, pdf)
Supplementary data
rmdopen-2022-002949supp001.pdf (301KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.