Pellagra Post–Roux-en-Y Gastric Bypass Surgery (original) (raw)
Abstract
Background/Objective
Micronutrient deficiencies such as pellagra are rarely seen after bariatric surgery and can be challenging to diagnose and manage. Alcohol use can precipitate nutritional deficiencies.
Case Report
A 51-year-old woman with a history of Roux-en-Y gastric bypass surgery who later developed an alcohol-use disorder after her diagnosis of breast cancer. She experienced a subacute decline in her physical and cognitive function along with a rash after radiation treatment for breast cancer, lower extremity pain and weakness, anemia, and diarrhea with severe hypokalemia. Workup showed undetectable niacin levels. She initially did not respond to an oral niacin replacement, necessitating intramuscular injections. Alcohol cessation and parenteral B complex replacement led to the resolution of her symptoms and biochemical derangements.
Discussion
Bariatric surgery with concomitant alcohol use can precipitate niacin deficiency–induced liver dysfunction. In the correct clinical setting, screening for alcohol use and checking niacin levels may help avoid extensive testing and can help make the correct diagnosis. Parenteral replacement may be necessary in this setting.
Conclusion
Niacin deficiency needs to be considered in patients with bariatric surgery with a history of alcoholism in the correct clinical setting.
Key words: nutritional deficiency, alcohol use, transaminitis
Highlights
- •
Screening for alcohol use in patients with a history of post–gastric bypass surgery can help diagnose potential complications such as micronutrient deficiencies - •
It is unclear whether screening for niacin deficiency in patients with a history of post–bariatric surgery is cost effective. However, checking niacin levels in patients with symptoms consistent with niacin deficiency is likely to be helpful - •
Intramuscular injection using B complex vitamins is likely to help correct the deficiency in patients with a history of gastric bypass surgery
Clinical Relevance
Bariatric surgeries are rising to counteract the obesity epidemic. Depression incidence is also high in obese patients. Depression can lead to increased alcohol consumption. This case highlights the risk of niacin deficiency in patients with a history of post–gastric bypass surgery who increase their alcohol intake because of worsening depression.
Introduction
We present the case of a patient with a history of Roux-en-Y gastric bypass (RYGB) in whom niacin deficiency developed after increased alcohol use. Niacin deficiency classically presents as diarrhea, dermatitis, and dementia, although presentations can vary. Bariatric surgery contributes to micronutrient deficiency, including niacin deficiency.1 Alcohol contributes to niacin deficiency by reducing absorption and decreasing the endogenous conversion of tryptophan to niacin.2 In the setting of both these comorbidities, it can be challenging to achieve the resolution of clinical symptoms with oral niacin, necessitating an intramuscular alternative in severe cases.
Case Report
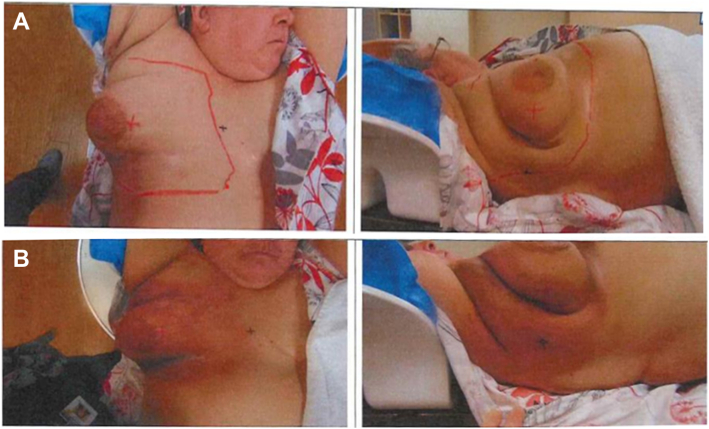
Our patient was a 51-year-old woman with a history of hyperlipidemia and morbid obesity who underwent RYGB in 2007 with a subsequent 100-pound weight loss. She did receive vitamin B12 injections subsequently as part of routine care, bariatric supplements, and vitamin D3 replacement. However, she developed persistent fatigue, and workup showed multifactorial anemia from malabsorption and heavy menstruation. She was treated with iron sucrose infusions as she was not initially tolerating oral iron supplementation. She also elected to undergo total laparoscopic hysterectomy with bilateral salpingo-oophorectomy. These interventions led to a significant improvement in her symptoms and resolution of anemia. She was diagnosed with invasive ductal carcinoma of the right breast in 2021(14 years after the gastric bypass) for which she underwent radiation therapy. Her mother had succumbed to breast cancer 20 years ago, and her diagnosis caused depression, leading to a significant increase in alcohol consumption. She received 16 rounds of radiation with a total radiation of 42.56 Gy. During radiation treatment, she developed a rash on her right anterior chest (Fig.).
Fig.
A, Before starting radiation treatment. B, After 16 rounds of radiation treatment. Erythematous dermatitis is localized to the radiation treatment area on the right breast.
Rash was limited to the radiation area, and the patient reported burning sensation initially and later progressing to a rough, scaly area of skin discoloration ranging from red to brown. The rash responded to treatment with topical steroids and emollient cream and cleared up 2 to 3 weeks following the completion of radiation treatment.
She did not require chemotherapy; however, a broad spectrum of symptoms and electrolyte deficiency developed after the completion of adjuvant radiation. She developed chronic diarrhea, imbalance, weakness with walking, leading to wheelchair use, bilateral lower extremity cramping and pain, and postmeal vomiting. Family members also noted a significant decline in patient cognitive functions. However, Montreal cognitive assessment testing was not performed. The patient who was working full time had to leave her job because of the above symptoms. The evaluation showed macrocytic anemia, mild leukopenia, and lymphopenia. She underwent upper and lower endoscopies, which showed mild gastritis. Bone marrow biopsy showed normocellular marrow with ringed sideroblasts. She also developed severe hypokalemia and hypomagnesemia, requiring oral potassium replacement.
She was found to have impairment in liver function with aspartate aminotransferase level ranging from 150 to 200 and alkaline phosphatase level ranging from 200 to 300. Extensive nutritional workup revealed zinc (59 μg/dL), tryptophan (8 μmol/L), and niacin (<20 ng/mL) deficiencies. Her copper levels were normal at 105 μg/dL (75-175 μg/dL). Plasma amino acid analysis showed a severe deficiency of tryptophan (Table 1).
Table 1.
Plasma Amino Acid Analysis
| Amino Acid | Reference Range (μmol/L) | Value | |
|---|---|---|---|
| Aspartic acid | 1-4 | 6 | H |
| Glutamic acid | 10-97 | 230 | H |
| Hydroxyproline | 4-27 | 14 | |
| Serine | 65-138 | 54 | L |
| Asparagine | 31-64 | 52 | |
| α-Aminoadipic acid | ≤2 | <1 | |
| Glycine | 122-322 | 128 | |
| Glutamine | 428-747 | 335 | L |
| Sarcosine | ≤4 | <1 | |
| β-Alanine | ≤5 | 3 | |
| Taurine | 31-102 | 41 | |
| Histidine | 60-109 | 100 | |
| Citrulline | 16-51 | 15 | L |
| Arginine | 43-107 | 20 | L |
| Threonine | 67-198 | 74 | |
| Alanine | 200-483 | 192 | L |
| 1-Methylhistidine | ≤47 | 4 | |
| γ-Aminobutyric acid | ≤3 | <1 | |
| Methyl Plus | 2-9 | 4 | |
| β-Aminoisobutyric acid | ≤3 | 3 | |
| Proline | 104-383 | 420 | H |
| Ethanolamine | 5-13 | 36 | H |
| α-Aminobutyric acid | 7-32 | 27 | |
| Tyrosine | 38-96 | 5 | L |
| Valine | 132-313 | 171 | |
| Methionine | 16-34 | 6 | L |
| Cystathionine | <1 | <1 | |
| Isoleucine | 34-98 | 28 | L |
| Leucine | 73-182 | 93 | |
| Homocysteine | <1 | <1 | |
| Phenylalanine | 40-74 | 44 | |
| Tryptophan | 40-91 | 8 | L |
| Ornithine | 27-83 | 61 | |
| Lysine | 119-233 | 36 |
Her medications at presentation included monthly B12 injections, calcium carbonate with vitamin D, and daily thiamine 100 mg. She was started on zinc supplementation and received an injection of vitamin B complex containing vitamin B1, B2, B3, B5, and B6 in the clinic, which she will continue receiving on a monthly basis. Interestingly, before obtaining the vitamin B complex injection, our patient was started on oral niacin 100 mg 3 times daily with little improvement in her symptoms. Repeat nicotinic acid levels after 2 months of oral therapy were still low (Table 2). She stopped consuming alcohol completely in June 2022. She was started on a high-protein diet as well.
Table 2.
Nicotinic Acid Levels Before and After Oral Therapy
| Reference Range | Before Treatment | On Oral Replacement | |
|---|---|---|---|
| Nicotinic acid (ng/mL) Nicotinamide (ng/mL) | See notesSee notes | <20<20 | <20 53 |
After 2 doses of intramuscular niacin, she had steady improvement in liver function tests and neurologic recovery with a return to an unassisted gait at her 3-month follow-up. There were no unanticipated or adverse events. Her anemia and electrolyte deficiencies also improved (Table 3). Patient cognition, balance, and gait speed also showed significant improvement.
Table 3.
Trend of Complete Blood Count, Electrolytes, and Liver Function
| Laboratory Value | Reference Range | Day − 30 | Day 0a Day + 7 | Day + 21 | Day + 60b | Day + 67 |
|---|---|---|---|---|---|---|
| Complete Blood Count | ||||||
| White blood cell count (×103/μL) | 3.7-11.0 | 2.0 | 2.3 | 2.9 | 5.6 | |
| Hemoglobin (g/dL) | 11.5-16.0 | 8.3 | 9.5 | 9.0 | 12.2 | |
| Platelets (×103/μL) | 150-400 | 117 | 221 | 235 | 319 | |
| Basic Metabolic Panel | ||||||
| Sodium (mmol/L) | 136-145 | 138 | 137 | 138 | 136 | |
| Potassium (mmol/L) | 3.5-5.1 | 3.3 | 2.8 | 3.5 | 4.3 | |
| Chloride (mmol/L) | 96-111 | 100 | 107 | 108 | 103 | |
| Bicarbonate (mmol/L) | 22-30 | 23 | 21 | 24 | 25 | |
| BUN (mg/dL) | 8-25 | 3 | 3 | 7 | 10 | |
| Creatinine (mg/dL) | 0.60-1.05 | 0.63 | 0.59 | 0.48 | 0.74 | |
| Magnesium (mmol/L) | 8.5-10.0 | 8.9 | 8 | 8 | 9.7 | |
| Phosphorous (mmol/L) | 1.8-2.6 | 2 | 1.6 | 1.7 | ||
| Calcium (mmol/L) | 2.4-4.7 | 1.8 | 3.5 | 4.5 | ||
| Liver Function Panel | ||||||
| Albumin (g/dL) | 3.5-5.0 | 2.2 | 1.7 | 2 | 3.5 | |
| Total bilirubin (mg/dL) | 0.3-1.3 | 7.7 | 11.4 | 2.4 | 1.1 | |
| Conjugated bilirubin (mg/dL) | 0.1-0.4 | 6 | 8.2 | 1.7 | ||
| AST (U/L) | 8-45 | 88 | 156 | 96 | 43 | |
| ALT (U/L) | 8-22 | 96 | 57 | 31 | 23 | |
| ALP (U/L) | 50-130 | 524 | 192 | 168 | 94 |
Discussions
Niacin is absorbed primarily from the duodenum and jejunum, with a small amount of absorption taking place in the stomach.3
Meat, dairy, and eggs are excellent sources of tryptophan. Dietary tryptophan is metabolized to niacin along the hepatic kynurenine pathway. Deficiency of niacin can therefore result from the consumption of a diet low in tryptophan, use of medications or drugs that block the conversion of tryptophan or niacin by blocking the hepatic kynurenine pathway, and malabsorption involving the stomach and small intestine.
Historically, poverty, famine, and war led to niacin deficiency as the affected population was forced to shift from meat to a maize-rich diet.2 Maize is a poor source of tryptophan; it contains a significant amount of niacin, but this niacin cannot be hydrolyzed by the mammalian digestive tract.4
With the increasing rate of morbid obesity worldwide, the number of people undergoing bariatric surgery has increased significantly. Bariatric surgery, while extremely helpful in achieving weight loss and reversing the metabolic problems associated with obesity, has some inherent risk of malabsorption of essential nutrients such as B complex vitamins, fat-soluble vitamins, copper, zinc, and iron.5 As highlighted above, niacin is absorbed from the stomach and small intestine, and its absorption will likely be affected by procedures such as RYGB and sleeve gastrectomy.
Short-term (<12 months) and long-term (>3 years) micronutrient deficiencies, including vitamin B complex vitamin deficiencies, were studied following laparoscopic sleeve gastrectomy (LSG) and RYGB surgeries. Interestingly, 12% of the patients were noted to have a niacin level of <55 μmol/L within 12 months after procedures, and almost 20 % of them had low niacin levels >3 years after surgery. No difference in niacin levels was found between LSG and RYGB patients in this study.5
Alcohol can decrease both the absorption of niacin and the conversion of tryptophan to niacin by inhibiting the tryptophan 2,3-dioxygenase enzyme. Patients with bariatric surgery who consume large amounts of alcohol are therefore at increased risk of niacin deficiency, both directly by reduced absorption and indirectly by the inhibited conversion of tryptophan to niacin. Our hypothesis for the subacute nature of her symptoms is that a significant increase in alcohol caused protein-calorie malnutrition causing low tryptophan levels and worsening micronutrient absorption.
Niacin deficiency is classically defined as a 3D syndrome with diarrhea, dermatitis, and dementia; however, the deficiency of niacin can vary significantly on a case-to-case basis, and a high index of suspicion is required in patients to make the correct diagnosis. Tryptophan is a precursor of serotonin, and therefore its deficiency can cause insomnia, anxiety, dysphoria, and psychosis. Confusion and memory loss can progress, leading to severe delirium, and stupor death.6
Especially in cases of alcohol-induced niacin deficiency, other features, such as hypoproliferative macrocytic anemia and leukopenia, are seen. Low albumin and elevated bilirubin levels are also been reported in alcohol-induced niacin deficiency.7 Severe diarrhea can induce hypokalemia and a low magnesium level. Colonoscopy with biopsy during the evaluation of postoperative diarrhea following bariatric surgery has shown inflammation with punctate erythematous erosions in the proximal colon and rectum, in a small series published by Kohli et al.8 Plasma niacin was found to be low in these patients with improvement in diarrhea on oral niacin treatment. Interestingly, pellagra rash is limited to a sun-exposed area; initially, this resembles a sunburn and then progresses to rough, scaly, hyperpigmented plaque. Our patient was predominantly homebound and the only major light exposure to her skin was during radiation treatment and the rash also improved 2 to 3 weeks following treatment, likely indicating the rash was precipitated by radiation therapy.
Treatment of alcohol-induced pellagra includes alcohol cessation and providing a diet rich in tryptophan and parenteral nicotinamide, which does not produce histamine release–related side effects of nicotinic acid. Because patients with alcoholism with bariatric surgery are likely to be malnourished and low in nutrients, it is a good idea to replace the vitamin B complex intramuscularly with other vitamins such as B2, B5, and B6.
This case highlights that niacin deficiency should be entertained in patients with bariatric surgery, especially with a history of alcoholism, in the setting of subacute neurocognitive, and functional decline.
Disclosure
The authors have no multiplicity of interest to disclose.
Acknowledgments
Patient Consent
Patient consent was obtained for this case report.
References
- 1.Punchai S., Hanipah Z.N., Meister K.M., Schauer P.R., Brethauer S.A., Aminian A. Neurologic manifestations of vitamin B deficiency after bariatric surgery. Obes Surg. 2017;27(8):2079–2082. doi: 10.1007/s11695-017-2607-8. [DOI] [PubMed] [Google Scholar]
- 2.Badawy A.A. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49(3):238–250. doi: 10.1093/alcalc/agu010. [DOI] [PubMed] [Google Scholar]
- 3.Pitkin R.M., Allen L.H., Bailey L.B., Bernfield M. National Academy Press; 2000. Dietary Reference Intakes for Thiamine, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. [PubMed] [Google Scholar]
- 4.Bender D.A. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101–197. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 5.Ledoux S., Flamant M., Calabrese D., Bogard C., Sami O., Coupaye M. What are the micronutrient deficiencies responsible for the most common nutritional symptoms after bariatric surgery? Obes Surg. 2020;30(5):1891–1897. doi: 10.1007/s11695-020-04412-8. [DOI] [PubMed] [Google Scholar]
- 6.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43(1):1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 7.Pinheiro H., Matos Bela M., Leal A.F., Nogueira L., Mesquita M. Hidden hunger: a pellagra case report. Cureus. 2021;13(4) doi: 10.7759/cureus.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli DR, Majithia R, Shocket D, Koch TR. The Potential Role of Niacin in the Development of Indeterminant Colitis After Bariatric Surgery. MedStar Washington Hospital Center. Paper presented at: MedStar Washington Hospital Center and MedStar Health Research Institute Research Day; July 5, 2013; Columbia, MD.