Past, Present and Future of the Aral Sea -A Review of its Fauna and Flora before and during the Regression Crisis (original) (raw)
Abstract
We review the past, present and possible future of the Aral Sea system in context of the human caused regression crisis that resulted in the drying out of the larger part of this original brackish water sea. The results are put into the context of other threatened saline lakes and the general water crisis in the world due to overexploitation of water resources and climate change. We cover the geographic history and hydrology from the origin of the sea 17,000 years ago to the present. The original biota including animals, higher plants and algae are covered in full detail, and tracked through the regression crisis. We put special emphasis on fish and fisheries because of their economic importance for the surrounding populations. We also review the side effects of the regression in terms of human health and changes to the terrestrial environment and local climate. We explain the dramatic improvements to the fauna in the northern Small Aral Sea following the construction of dams to retain its waters and discuss future options to further improve this restored water basin. We contrast this with the progressing hypersalinization of the remnants of the southern Large Aral Sea, which faces conditions that will eventually render a “Dead Sea” condition hostile to all metazoan life. We end by highlighting the partial restoration of the Small Aral Sea as an example of how much restoration can be achieved for relatively little financial expense and in a short period, when good ideas, kind hearts and hard work operate together for the benefit of the environment and our human society.
Keywords: Global Change, Ecology, Saline lake, Fisheries, Irrigation, Agriculture
BACKGROUND
Water bodies in arid areas around the world are facing serious problems, mostly due to increased diversion of water for human purposes such as irrigation of agricultural fields. This situation is now exacerbated by ongoing global warming, which has caused water bodies to experience decreased levels of precipitation and water flow in rivers (see_e.g._, López-López 2021). Almost all affected water bodies, whether rivers, freshwater lakes or saline lakes, have a high biological value in terms of biodiversity and ecosystem services, including the economy and lifestyle of local human societies (Nature Editorial 2023). Prominent examples are the The Great Salt Lake (Oren 2018; Kintisch 2022; Derouin 2017), Colorado River (Stokstad 2021; Fleck and Udall 2021), Lake Tchad (Nour et al. 2020; Pham-Duc et al. 2020), Lake Urmia in Iran (Oren 2018; Radmanesh et al. 2022; Davarpanah et al. 2021; Hobbins and Barsugli 2020), the River Jordan (Katz 2022; Givati et al. 2019) and Lake Balkhash and the Aral Sea in Central Asia (Mischke 2020). The most serious case is the virtual desiccation of the larger part of the Aral Sea (Figs. 1, 2). This crisis has been well documented in specialized journals and easily ranks as the most serious local area environmental disaster in recent times (e.g., Deliry et al. 2020; Loodin 2020; Micklin 2007 2016; Micklin et al. 2014 2020). It has even been subject to personal visits by two General Secretaries of the United Nations, Ban Ki-Moon and Antonio Guiterres, the former calling the shrinking of the Aral Sea “one of the planet’s worst environmental disasters”. (The Telegraph 2010; UN News 2010; Agency of IFAS 2022). Yet, unlike the vanishing rain forests or damage to the coral reefs, the Aral Sea crisis has yet to gain full international attention. To illustrate this, a search in the Web of Science (title field; core collection; last five years) offered 260 hits for the Colorado River but only 144 for the Aral Sea. Even more critically, limiting the search to just the journals Nature and Science offered only a single hit for the Aral Sea but five for the Colorado River.
Fig. 1.
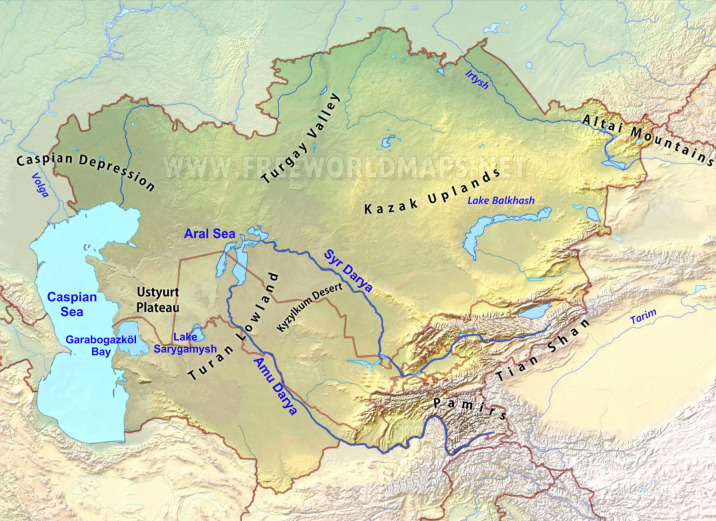
The position of the Aral Sea in Central Asia. The only water inflow is from the southern Amu Darya and the northern Syr Darya rivers, which originate in the Pamir and Tien Shan Mountains. The Aral Sea is depicted with its present, highly reduced extension. Modified from www.earthmaps. org and Plotnikov et al. (2021b).
Fig. 2.
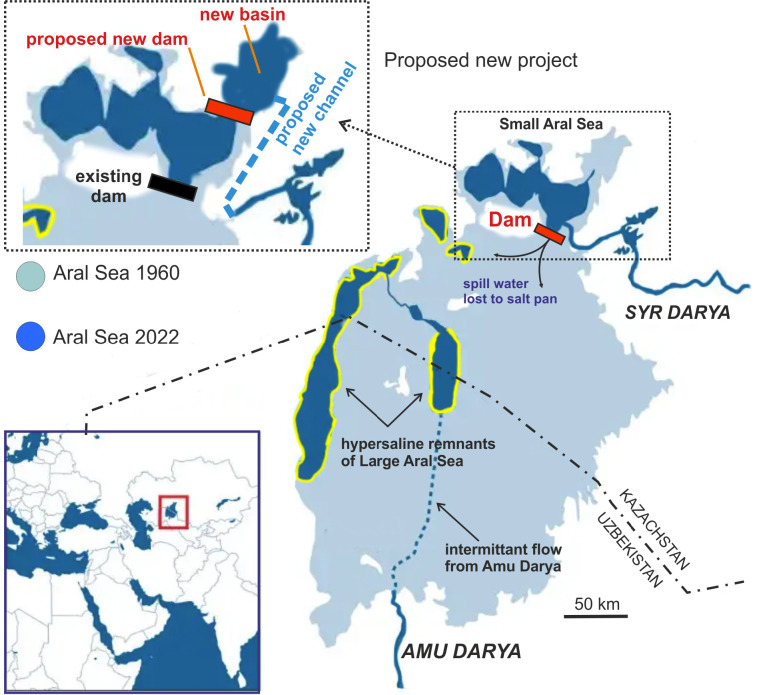
The Aral Sea before the regression and now. Light blue shows the original sea; dark blue is the present extension. The variable shorelines of the hypersaline Large Aral is shown in yellow. The inset at upper left shows the existing Kokaral Dam and plans for an additional dam to restore even more of the Small Aral Sea. Based on Plotnikov et al. 2021b.
Diversion of water from its only two inflowing rivers caused the Aral Sea to regress in surface area from 67,499 km2 in 1960 to only 39,734 km2 in 1990. This entailed increasing salinity and a gradual disappearance of most of the original biota (Aladin and Potts 1992; Aladin et al. 2019). By 1990 the original Aral Sea had separated into a northern Small Aral and several isolated water bodies in the south, all the latter rapidly becoming hypersaline and unliveable for most metazoan life forms. Commercial fisheries, formerly an important occupation and source of food protein in the area, virtually ceased (White 2014). Adding to this, the vanished sea caused the local climate to become more violent and unhealthy (Deliry et al. 2020). Altogether, the Aral Sea crisis entailed a multitude of interrelated problems that seriously affected the economy, health and livelihood of the surrounding population.
In 1992, a first primitive dam was constructed to retain water in the northern Small Aral. When damaged due to bad weather, The World Bank financed a much more robust replacement that, since 2005, has ensured a positive water balance in this water body. Soon after, many animals reappeared in the Small Aral and fisheries rebounded (Micklin 2016; Micklin et al. 2020; Plotnikov et al. 2016). By contrast, the southern Large Aral developed into several more or less water isolated bodies that all became hypersaline and increasingly hostile to most life forms.
Here we review the history, hydrology and biology of the Aral Sea before, during and after the human caused regression crisis. We cover all relevant fauna and flora elements, including invertebrates, fish, waterfowl, microalgae and macrophyte vegetation in the sea and along the shores. We put special emphasis on the commercial fisheries due to their economic and social importance in the area. We also discuss the human health issues and effects on terrestrial ecosystems that were intrinsically linked to the former Aral Sea.
MATERIALS AND METHODS
General aspects of the Aral Sea
Location, topography and climate
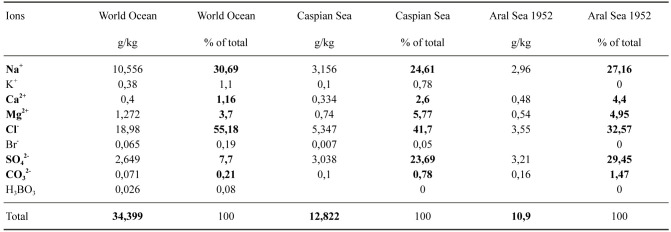
The original Aral Sea was a large body of saline water located in an area below sea level in Central Asia (Fig. 1). Its topographical parameters are given in figure 2. Before its modern regression, it was the second largest lake in the world by area (Plotnikov et al. 2021a). The Aral Sea has no effluents, and the only inflowing water comes from the Amu Darya in the south and the Syr Darya in the north. These two rivers originate in the Tien Shan and Pamir mountains (Fig. 1). Like the Caspian Sea and many smaller water bodies in Central Asia, the Aral Sea was brackish with an original mean salinity at 10.3 grams/kg. It is noteworthy that its composition differed from sea water in being enriched in divalent ionic forms (Table 1).
Table 1.
Ionic composition of the Aral Sea before the Caspian Sea. Note the relatively high values of divalent cations (Ca2+, Mg2+) and anions (SO42-, CO32-) in both the Aral Sea and the Caspian Sea. Data from IFAS (Agency of IFAS for implementation of the Aral Sea basin)
The Aral system is located in an arid continental area with an original seasonal temperature range of -18 to 35°C in the north (Aralsk) and -12 to 34°C in the south (Muynak). The large surface area of the original sea acted as a buffer on seasonal temperature oscillations. Still, the lake became ice covered in the winter, while evaporation was substantial during summers, but until the mid 20th century the water balance was nonetheless fairly stable.
Topographically the Aral Sea consisted of a northern Small Aral and a southern Large Aral, these being connected by two narrow straits on either side of the Kokaral Island. There were several other variously sized islands, especially those forming the Akpetkinskyi Archipelago at the southeastern end.
Habitats
The original Aral Sea offered considerable diversity in habitat, thus promoting biodiversity. The sea at large was brackish, but there were large areas close to the two river deltas that were connected to the pure fresh water in the rivers themselves. Alongside the lower part of the rivers, there were also numerous, essentially isolated lake systems with almost fresh water (Plotnikov et al. 2021b). In contrast, there were also shore locations that sustained a permanent or temporarily increased level of evaporation, thus causing a higher salinity than in the sea at large. Hence, the entire Aral Sea offered a salinity range from saline over brackish to fresh water and from lacustrine to riverine habitats. The habitat diversity was utilized by the native ichthyofauna. Many species migrated to the shore or into the rivers for breeding, since their fry could not tolerate the saline waters. Finally, close to the sea there were also smaller hypersaline water bodies, isolated from but faunistically connected with the Aral Sea itself due to the spread of organisms by animal or wind transport (Plotnikov et al. 2021b). Both the river deltas and their associated lake systems became pivotal refugia that enabled many species to survive the height of the regression crisis and eventually enabled repopulation of the restored Small Aral.
Origin of the Aral Sea
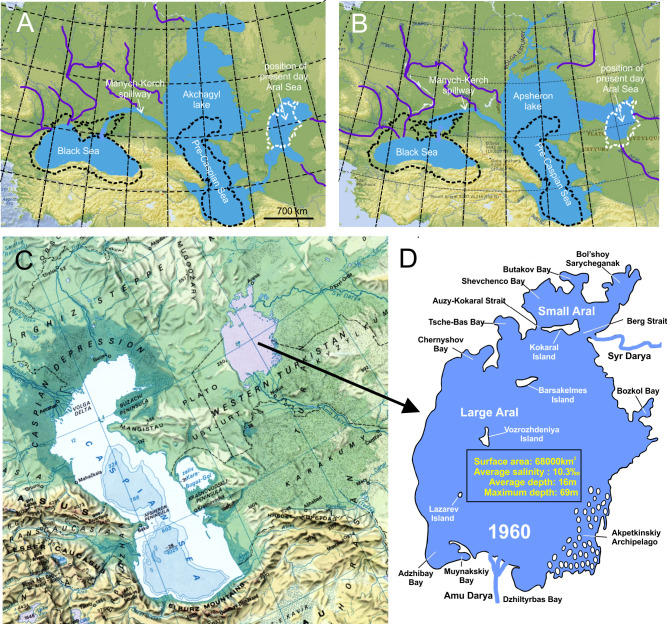
The modern view of the history of the Aral Sea is different from how it was presented in the past (Boomer et al. 2009; Svitoch 2010; Burr et al. 2019; Krijgsman et al. 2019). The present sea arose ca. 17,000 years ago from water that flowed into a dry depression. During the Pliocene, a drain-less depression already existed in the place of the modern Aral Sea. It was formed in an arid climate as a result of deflation,i.e., the process of wind blowing loose particles off rock surfaces. In the late Pliocene, during the transgressions of the Caspian, the Aral Sea depression was filled with waters first of the Akchagyl Sea and later of the Apsheron Sea (Fig. 3; Zonn 2009). The vast Akchagyl Sea (or Basin) covered both the present Caspian and Aral Sea and an extensive area between the lower Volga and Ural rivers. The Akchagyl Sea was a deep water, saline and cold basin with a water level 100–150 m above the present Caspian Sea. Some of its fauna intruded into the Black Sea with which it was connected by the Manych-Kerch Strait (Fig. 3). The later Apsheron Sea existed during late Pleistocene and was smaller than the Akchagyl Sea, but larger than the modern Caspian Sea (Krijgsman et al. 2019). Eventually losing the connection with the Black Sea, the Apsheron Sea became shallower, warmer and also less saline (salinity similar to the present Caspian Sea).
Fig. 3.
A and B, Geological history and topographic details of the Aral Sea from late Pliocene to present; the extensions of the present Black Sea, Caspian Sea and Aral Sea are shown in outline. The large Akchagyl Sea (or Basin) (~3.2–2 mln. years BP) covered a vast area and connected to the Black Sea by the Manych-Kerch Pillway. The later Apsheron Sea (~1.8–0.7 mln. years BP) was smaller, but still larger than the present Caspian Sea. C, The position of the present Aral Sea in its extension before the modern regression. D, Topographic and hydrological details of the Aral Sea as of about 1960. Original figure partially based on Krijgsman et al. (2019).
These events were important for the fauna of the present Caspian Sea, but not for the Aral Sea, which dried up completely during a following continental period that lasted almost until the end of the Pleistocene (Burr et al. 2019; Aladin and Plotnikov 1995; Svitoch 2010). The waters of the Syr Darya started flowing into the Aral Basin from the Late Pleistocene. Subsequently, Amu Darya also turned to flow into the Aral Sea rather than to the Caspian. Accordingly, the Aral Sea biota consist of invaders that entered it at different times and from different faunal provinces.
From its formation 17,500 years ago, the Aral Sea has experienced repeated regressions and transgressions (Leroy et al. 2007). The water level and salinity of this drainless basin were influenced only by climate and the precise course of the Syr and Amu Darya. Climate, drier or wetter, determined both loss to evaporation and water flow in the rivers from their sources in the Pamir and Tien Shan mountains. But while the waters of the Syr Darya always flowed into the Aral Sea, the Amu Darya could at times flow into Lake Sarygamysh to the south-west of the Aral and further along its ancient channel –Uzboy –into the Caspian Sea (as was the case in the Pliocene). Alternatively, it could also flow simultaneously into both reservoirs. Eventually, the amount of inflowing water was also affected by the emergence and development of irrigated agriculture. During the Khwarazmian Dynasty (1077 to 1231) people could actually shift the flow of the Amu Darya from the Aral to the Caspian, or vice versa, but such control could only be maintained during periods of relative social affluence and stability. Social upheavals and wars in the region, such as the Mongolian invasion, lead to the loss of control over the river. Protective dams and irrigation systems were destroyed, and then, by chance, the flow of the Amu Darya turned in one direction or another (Aladin and Plotnikov 1995).
The variability in size of the Aral has been well-documented, although until recently the exact water levels have been debated (Boomer et al. 2009). Even in medieval Arabic documents there are references to changes in water level and direction of flow of the Amu Darya. When inflow decreased, the Aral Sea would sometimes break up into separate lakes filled with highly mineralized water, while near the river mouths there were floodplains with freshened shallow waters (Svitoch 2010). Dating of the regressions and transgressions over the past 2000 years are based on data from geology, geomorphology, archaeology and on fossilized remains of aquatic organisms in bottom sediments (Boomer et al. 2009). The first regressions are dated approximately from the 1st century BC to the 4th century BC. Regressions during the last millennium have been dated more precisely and were also documented in contemporary historical records (Krivonogov 2014; Krivonogov et al. 2010 2014; Yang et al. 2014). The data of Boomer et al. (2009) indicate that the Aral Sea experienced minima in area and level between AD 900–1350, AD 1500–1650 and 1790 to the present. Nevertheless, all records indicate that a large scale and very rapid regression started during the early 1960s and was almost exclusively due to large-scale diversion of upstream waters from the Amu Darya for agricultural purposes (Boomer et al. 2009; Aladin and Potts 1992; Micklin 2007). This eventually resulted in the present state, where the Aral Sea has been reduced to only a tiny remnant of its former size.
In summary, the Aral Sea is a comparatively young system, whose inflowing rivers varied in water volume and also changed course since the Ice Ages, thus together affecting its area, water level and salinity. It always remained isolated from any other large water bodies such as the Caspian Sea. This entailed a low biodiversity, and its young age also means that it contained few endemic forms. We emphasize that despite variations in the past, the Aral Sea never experienced any condition remotely resembling the severe, human caused regression that occurred during the latter half of the 20th century and eliminated most of the original sea and its biota.
Investigations of the Aral Sea
The Aral system was an early target for detailed biological investigations, starting with the efforts of Berg (1908). The importance of this multidisciplinary scientific study of both the Aral and other areas in Central Asia can hardly be underestimated (Goaravetisyan 2021). During the latter half of the 20th century, it was subjected to detailed monitoring of physico-chemical and biological parameters. These took place regularly and at fixed stations by local researchers and staff from the Zoological Institute, Russian Academy of Sciences (ZIN RAS). As a result, both the original state and the entire period of regression have been very well documented (details in Plotnikov et al. 2021b). The monitoring even included screening of cores taken from the dried out lake bed (thanatocoenoses) that allowed establishing time series of organisms that left hard identifiable parts, such as Ostracoda (Aladin 1991).
RESULTS
Original biodiversity
We here review the native aquatic fauna and flora and their decline during the regression period. We include important invertebrate taxa, fish, water birds, microalgae and macrophytes, including both macroalgae and flowering plants in the sea and along the shores. The native fauna has previously been surveyed by Aladin and Potts (1992) and also reviewed briefly in Keith et al. (2013), but here we add new and more detailed information. The crustacean and fish fauna was previously treated in detail (Ermanakhov et al. 2012 2013; Plotnikov et al. 2021b). According to our most recent estimate, the native aquatic fauna of the Aral Sea comprised 20 species of fish, 195 species of free-living invertebrates and 71 species of parasites (Fig. 4). For the ichthyofauna, the most important food items were benthic and planktonic crustaceans and the benthic bivalves and chironomid larvae. In addition to the aquatic fauna, there were a number of terrestrial animals, especially birds, closely associated with the Aral Sea. The diversity of microalgae was impressive with more than 640 species. They served as food for invertebrates both in the plankton and on bottom sediment and plant surfaces. The flora of macrophytes contributed to habitat formation both along the shores and in deeper waters. It comprised 24 species of angiosperms, six species of charophytes and about 40 other species of macroalgae. Some macrophytes were also very important as food items for omnivorous fish and some of the waterfowl. For invertebrates, our main focus is on crustaceans and molluscs, which were the principal food items for the fish.
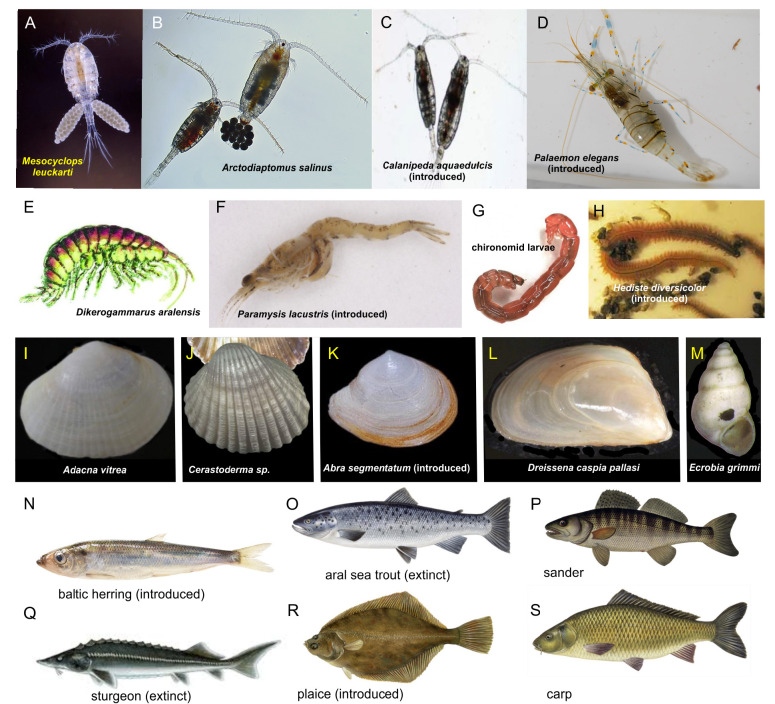
Fig. 4.
Aral Sea Fauna. Important native and introduced species in the Aral Sea fauna.Mesocyclops leuckarti and Arctodiaptomus salinus were important members of the zooplankton, but the latter was replaced by the introduced Calanipeda aquaedulcis. In the benthos_Dikerogammarus aralensis_ was displaced by the introduced shrimp_Palaemon elegans_. Bivalves of the genus Adacna,Cerastoderma and the introduced species Abra segmentum were important food for fish. The same is true for the introduced polychaete_Hediste diversicolor_. The fish depicted were all valuable commercial fisheries. Chironomid larvae and pupae were important food items for fish. The Aral Sea Trout and the Aral Sea Sturgeon have both gone extinct. Further details in text.
Invertebrates
Crustaceans and molluscs were the most important invertebrates in the original Aral Sea, being primary food items for most native fish (Aladin et al. 2022). In table 2, data are given for all native species of Mollusca. The Crustacea were previously given detailed attention (Aladin et al. 2021; Plotnikov et al. 2021b) so information on this group is only summarized at higher taxon level (Table 2).
Table 2.
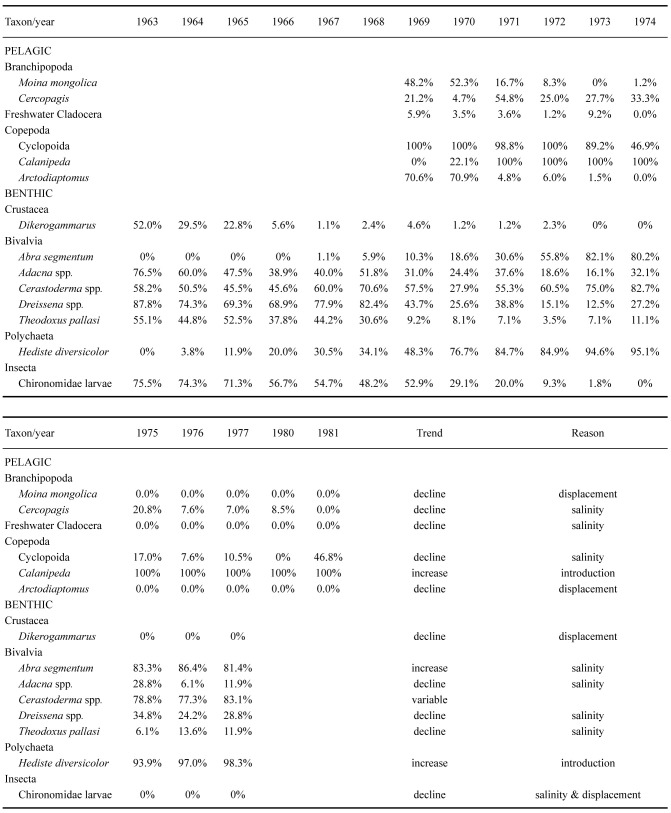
Occurrence of selected invertebrate taxa at fixed stations during the regression crisis. Percentage of total stations where taxon was found are indicated (See Plotnikov et al. 2021b for details). For Crustacea, a complete species list appeared in Plotnikov et al. (2021b) so data is only summarized for genera or higher taxa
Native crustaceans comprised species with varying degrees of salinity tolerance, which affected their distribution. Nine species of pelagic cladocerans were present in the plankton, but they were always restricted to areas with low salinity. The plankton also contained two species of calanoid copepods and 14 species of cyclopid copepods. Especially important as fish food were the euryhaline Arctodiaptomus salinus and Halicyclops rotundipes, which were both found throughout the sea. On or associated with the bottom were 15 species of Harpacticoida and 11 species of Ostracoda, again with varying degrees of salinity tolerance. The euryhaline gammarid Dikerogammarus aralensis was the only native malacostracan. Finally, there were five species of parasitic copepods hosted by the native fish fauna.
Native Mollusca were poorly represented compared to Crustacea, but they still provided an important food source for fish (Aladin et al. 2022). The literature has been troubled with incorrect naming of species, but here we follow the most recent and authoritative account by Wesselingh et al. (2019). The Aral Sea contained two species of Gastropoda (Ecrobia grimmi, Theodoxus pallasi) and seven species and subspecies of Bivalvia, including two species of_Cerastoderma_, three subspecies of Dreissena and two subspecies of Adacna. Among the bivalves, Adacna minima minima lived throughout the sea even down to 30 m while other forms were most numerous at shallow depths. The two species of Cerastoderma differed in distribution, with the saltwater tolerant C. glaucum occurring in the more saline areas. The other species was previously identified as C. rhomboides, but is here called C. sp. A. (Wesselingh et al. 2019), occurring only in the lower salinity waters. All the bivalves reproduced during the summer, at which time their larvae were the most numerous component of zooplankton. (Lukonina 1960; Kortunova 1975). Initially the fish intensively ate only the two widely distributed subspecies of Adacna minima, while consumption of_Cerastodema_ spp. and Dreissena spp. of older ages were limited due to their thick shells. After being introduced in 1960–1963, the Mediterranean-Atlantic mollusk Abra segmentum, which also has a thin-walled shell, became an additionally valuable food source for the benthivorous fish (Karpevich 1960 1975; Yablonskaya 1960).
Chronomid larvae were a significant element in the deep water bottom area, and provided an important food item for fish when they swarmed to the surface and depupated into imagos. The strict seasonal availability of this food item together with the generally low density of free water crustaceans explains why none of the native fish were exclusive plankton feeders.
Meiobenthos have not traditionally been studied in the Aral Sea, but recently living samples were collected in Large Aral Sea in 2003 and 2004 at depths of 0 to 39 m (Mokievsky 2009; Mokievsky and Miljutina 2011). In the now hypersaline waters, the near-bottom salinity at the sampling sites varied from 88 to 109‰, but there was still a diverse meiobenthos consisting of nematodes, harpacticoids, ostracods, turbellarians and foraminiferans. The density showed significant spatial variation, with nematodes predominating in most samples. The maximal abundance of free-living nematodes (1440 specimens/10 cm2) was recorded in 2003 at a sampling site at 10 m depth, 89 ppt salinity and 13.6°C. In 2004, the maximal abundance of nematodes was 750 specimens/10 cm2 at about the same depth and salinity, but at 24.5°C. The highest value for harpacticoid copepods was 116 specimens/10 cm2 at 1 m depth. The high spatial variation in meiobenthos density is to a considerable extent related to the sediment characteristics at the sampling stations. Meiobenthos is now often used for biomonitoring purposes and is normally dominated by nematodes in terms of specimen numbers (Semprucci et al. 2015). For comparison with the Aral Sea, Huys et al. (1992) sampled meiobenthos all over the North Sea and found that harpacticoids were almost always less frequent than nematodes, whose density ranged from 61–4167 individuals/10 cm2. The density of meiobenthos in the hypersaline parts of the Aral Sea agrees well with other studies. In marine habitats, conditions can sometimes also reach hypersaline levels, but if they exceed 100 ppt, all macro-infauna vanishes, while meiofauna remains at relatively low densities. In tidal areas off Zanzibar, Olafsson et al. (2000) found salinities as high as 89–160 ppt in sediment pore waters and specimen densities in the meiobenthos at 271 to 656 specimens/10 cm2, the majority of these (58–87%) being nematodes.
Native ichthyofauna
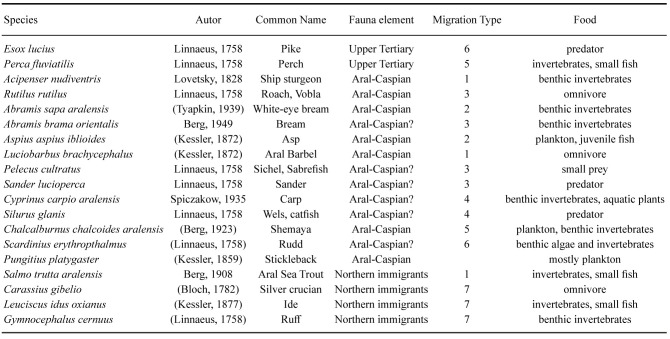
The native ichthyofauna of the Aral Sea comprised only 19 species from seven families; 12 from the Cyprinidae, three from the Percidae while Acipenseridae, Salmonidae, Siluridae, Esocidae and Gasterosteidae were represented by one species each (Ermakhanov et al. 2012). Most of these fishes were eurybiontic, i.e., tolerating a wide range of environments (Nikolsky 1940). The original ichthyofauna belonged to three faunistic complexes: Upper Tertiary fauna, Aral-Caspian Fauna and Northern Immigrant Fauna (Table 3).
Table 3.
Native Ichthyofauna of the original Aral Sea. The migration types are explained in the text. Taxonomic names rely on information from FishBase (fishbase.se) and World Register of Marine Species (WoRMS)
The Aral-Caspian fauna comprised nine species that inhabited both the sea itself and the lower reaches of the tributary rivers. The northern immigrants consisted of mainly Siberian fishes. Among these, the now extinct Salmo trutta aralensis (Aral Sea Trout) was a stenothermal and low temperature tolerant species. The remaining northern immigrant species are eurythermal and limnophilic, and they inhabited the lower reaches of the rivers and were found partially across the Aral Sea.Carassius gibelio (silver crucian) and Leuciscus idus oxianus (ide) lived only in the freshened parts of the Aral, while Gymnocephalus cernuus (ruff) was found in both saline and fresh water parts. The young age of the Aral Sea as an isolated water body probably explains why there were no endemic genera or species, endemism existing only at the subspecies level.
Migration, spawning and recruitment
Most species were migratory (Table 3, Fig. 5). This concerned migrations of juveniles from spawning areas to deeper stations, migrations of adult fishes to spawning areas and their return to foraging areas and migrations to the places of overwintering. No species reproduced in the pelagic or remained in deep waters for their entire life. This testifies to the Aral Sea fish fauna originating from the limnophilic fauna of the Amu Darya basin (Nikolsky 1940). Based on migratory patterns, Nikolsky (1940) recognized seven different groups among the fish (listed 1–7 in Table 3).
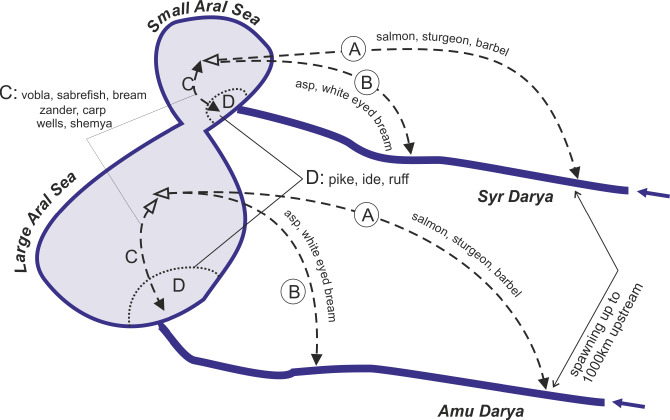
Fig. 5.
Highly schematized representation of reproductive patterns of native fishes in the Aral Sea. A–D summarises the migratory patterns explained in detail in the text. A, Long distance migration into the rivers for spawning. B, Shorter distance migration into rivers for spawning. C, Migration from open sea to shallow coastal waters for spawning. D, Fish that stay and reproduce near the shore.
Group 1 were anadromous fishes that spawned in the two rivers, sometimes as the now extinct Acipenser nudiventris (ship sturgeon) more than 1000 kilometers upstream. Most entered the rivers in the summer but did not reproduce until the following year. Foraging areas were located in the sea outside the influence of fresh riverine water.
Group 2 were semi-anadromous fishes that entered the rivers for spawning in the spring but never migrated very far upstream. Among these Abramis sapa aralensis (white-eye bream) fattened off-shore, while Aspius aspius iblioides (asp) fattened near the coast.
Group 3 were fish that twice a year came up to the coast from the open sea; in the spring for spawning and in autumn after having fed during the summer in deeper waters. Their spawning grounds were located both in the freshened or higher salinity coastal areas. Abramis brama orientalis (bream) also migrated for spawning into lakes located along the lower parts of the river, while Sander lucioperca (sander) spawned only in the rivers. Rutilus rutilus (vobla and roach) never entered the rivers. The most commercially important species (vobla, bream, sabrefish, sander) belonged to this group or (carp) to group 4.
Group 4 spawned among vegetation in the rivers and the coastal zone, the main fattening areas were near shore.
Group 5 comprised only Chalcalburnus chalcoides aralensis (shemaya), which once a year migrated to freshened or saline parts of the coastal zone and after spawning returned to the open sea. It did not enter the rivers.
Group 6 remained constantly in the coastal zone, both saline bays and freshened areas of deltas, where they inhabited thickets of vegetation. This group also consisted of reed forms of roach, bream, carp, and wells (catfish). No migration.
Group 7 fishes remained in freshened areas of deltas and lower rivers and deltatic lakes. No migration. The original fish fauna consisted of species that
typically reproduced in fresh water. They migrated for spawning from the open sea to the coastal zone or into the rivers or they remained constantly and spawned in fresher zones near the coast. This biology is important both for understanding the regression crisis and for efforts at restoring the Aral Sea. The role of spawning in saline waters has remained unclear. Bream, carp and roach can spawn at a wide range of salinities from fresh to full saline waters, but this does not mean that embryogenesis and larval development will proceed normally at increased salinities. Available data on the upper salinity thresholds for normal development is inconsistent. Experimental data suggest that, for roach, the upper bound is equal or close to the full salinity of original Aral Sea waters, but lower for carp and bream (Konovalov 1950). On the other hand, there were schools of bream near the western coast and thus far from deltaic areas, and here this species spawned in full saline waters (Bervald 1950). There have also been observations of normal development of roe and larvae of Aral roach, bream and carp at salinities in the 10–12 ppt range (Gosteeva 1956 1957 1959).
Fishes from the open waters also differed in patterns of diurnal vertical migration (Nikolsky 1940). During the entire period of plankton blooming, sabrefish and shemaya swam to surface layers at night and descended during daytime, passing the thermocline during these vertical migrations. Roach and white-eye bream had similar vertical migrations, but only during the spring and autumn during vertical water circulation, while they remained in the hypo-limnion during summer stagnation. Other fish, such as bream remained in the deeper waters during the entire blooming period. Finally, sander had a random pattern of vertical distribution, irrespective of time and temperature stratification.
Feeding and food items. In the open part of the Aral Sea, bream, roach, sander, white-eye bream, sabrefish (Pelecus cultratus) and shemaya foraged (but never spawned) from the second half of May until October. During summer, these fish remained and fed all day near the bottom. Feeding types comprised predatory fishes, planktivores and benthos feeders, with some feeding on both plankton and benthos. There were four species feeding principally on zoobenthos, and the principal food items were the amphipod Dikerogammarus aralensis, bivalve mollusks and the very abundant chironomid larvae (Nikolsky 1940). In the open water and at the surface, important food items were pupae of insects, mainly of Chironomidae, floating to the water surface during their mass flying out, and also chironomid and caddis fly imagos. When seasonally available, many benthic feeding fish would shift to this food source, especially sabrefish, shemaya, roach and white-eye bream (Nikolsky 1940). Phytoplankton (blue-green, green and diatoms) were essential food for juveniles of some fish –most of all shemaya (diatoms, blue-green), vobla (diatoms, green –Spirogyra) and bream (diatoms) (Pankratova 1935; Nikolsky 1940). For older and adult fish, the role of zooplankton was low, and none of the native fish in the open waters depended entirely on this food source. Only the shore inhabiting stickle-back (Pungitius platygaster) depended entirely on zooplankton. The coastal zone was more diverse in terms of available food items and feeding patterns, and the role of plankton was much less than in open waters. Near shore were six species of primarily zoobenthophages, e.g., bream, white-eye bream, aral barbel (Barcus cyclolepis) perch, roach and carp. Bivalve molluscs were their main food (Aladin et al. 2022), with the gammarid D. aralensis and ostracods also being important. Rudd and carp also fed on macrophytes. Predatory species were zander, pike and wells. For roach that were found both on and off shore, the role of vegetation as food was higher in the coastal zone.
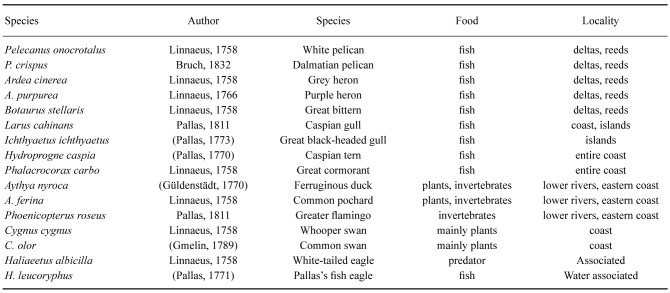
Birds
A large number of birds were and continue to be associated with the Aral Sea and river systems (Micklin et al. 2014). The majority of these were migratory water birds, since the iced over waters offered no options for such species during winter (Table 4). They therefore utilized the Aral Sea area as a stop for resting and feeding during the spring and fall migrations. Many birds also had nesting grounds on floating islands at the shore, in reed thickets in the river deltas and in the shallows of the Aral itself, primarily in its freshened zones. Fish and aquatic invertebrates were the main food sources. Seagulls and terns also fed on small rodents. Two swan species consumed aquatic plants as their primary food source. Several of the birds, such as flamingos, are threatened species that also have a very high potential for attracting ecotourists.
Table 4.
Important water associated birds of the Aral Sea
Microalgae
In the Aral Sea microalgae occurred not only in phytoplankton, but were also abundant as phytobenthos and periphyton, and in all these habitats they served as food for invertebrates. Diatoms (Bacillariophyceae) predominated both as plankton and on surfaces The first investigations on planktonic microalgae algae were by Borshchov (1877) and Berg (1908). Since then, the number of known species has increased rapidly (Ostenfeld 1908; Behning 1935), but more recent general surveys are scarce. Rusakova (1995) provided a very detailed account after the Aral Sea separated into two water bodies, but since then there have been no studies of microalgae in the Small Aral Sea. More recently, microalgae were studied in the now hypersaline Large Aral by Sapozhnikov et al. (2009) and Zhitina (2011).
To date over 650 species and intraspecies of planktonic and benthic microalgae are known to exist or have existed in the Aral Sea. The diversity of phytoplankton in the Tshche-Bas Bay (Large Aral) and the Shevchenko and Butakov Bays (Small Aral) is much lower than in the freshened area of the sea, primarily due to the almost complete loss of green algae. For our account of both micro-and macroalgae we followed the names given in Algaebase (https://www.algaebase.org/).
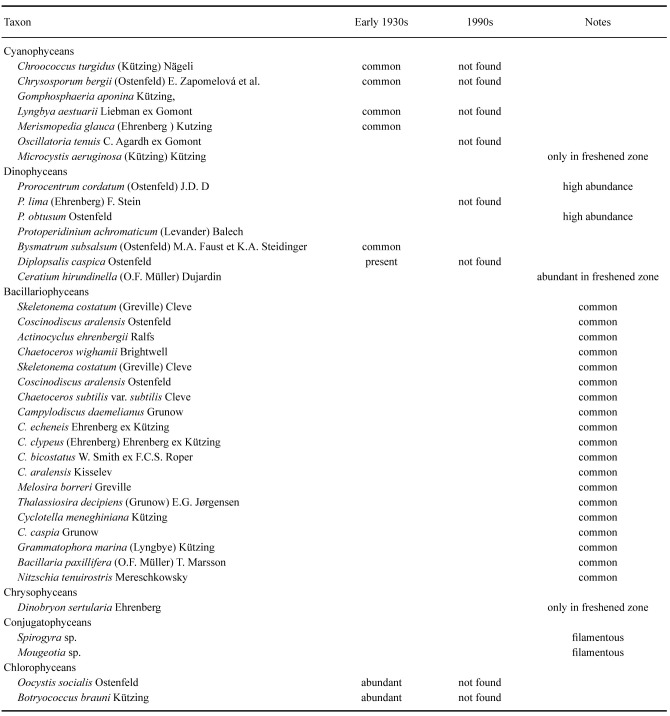
Both in the past and at present, diatoms (Bacillariophyceae) make up approximately 2/3rds of the total species diversity of microalgae and dominate in both plankton and on sediment and macrophyte surfaces. Either on surfaces or in the plankton occurred species of genera such as Amphora, Cocconeis, Cymbella,Diploneis, Epithemia, Gomphonema,Gyrosigma, Navicula, Nitzschia,Pinnularia, Pleurosigma, Surirella and Tryblionella. Diatoms are followed in abundance by several dozens of species of Cyanophycea, Chlorophycea, and Dinophycea. Finally, there occur a few species of Chrysophycea, Dictyochophycea, Cryptophycea, Pedinophycea, Prasinophycea, Ulvophycea, Conjugatophycea, Klebsormidiophycea, and Euglenophyta. Table 5 lists some of the more important microalgae, including those recorded in the early 1930s (Behning 1935) and from studies after 1990 when the regression crisis had culminated. Details from these later studies are summarized below with respect to the different parts of the Aral Sea.
Table 5.
The more abundant microalgae found in the Aral Sea in early 1930s (Behning 1935) and those found in the 1990ties later after the regression crisis had culminated
Tshche-Bas Bay of Large Aral had a salinity of 41 ppt in 1992 and the poorest phytoplankton diversity. Highly abundant were various species of the genus_Chaetoceros_ –C. wighamii, C. subtilis var. subtilis, C. socialis f.socialis, considered to be brackish-marine euryhaline species. Representatives of brackish-water forms of dinophyte algae were noted in large numbers –Prorocentrum cordatum, P. obtusum, Goniaulax apiculata, G. spinifera, Glenodinium lenticula f. lenticula, G. pilula, as well as the marine euryhaline diatoms Actinocyclus ehrenbergii and Cocconeis scutellum. Of the blue-green algae were found, Spirullina labyrinthiformis and Johannesbaptistia pellucida f.anabaeniformis, both characteristic of brackish and salty shallow areas of inland seas and water bodies of Central Asia. The only species of green algae,Oocystis borgei var. borgei, was extremely rare. From the total number of identified species, there are varieties and forms of mesohalobes (13), marine euryhaline (7), indifferent (7) and halophiles (7) (Rusakova 1995).
Butakov Bay of the Small Aral had a salinity of 36 ppt in 1992. Diatoms (Bacillariophycea) recorded here were Cocconeis scutellum,Cyclotella menighiniana, Diatoma tenue var.elongatum, Cymbella ventricosa, Gomphonema ventricosum, G. olivaceum, Tryblionella acuminata W. Smith, and the brackish marine planktonic species Achnanthes brevipes. Representatives of the Dinophycea were Prorocentrum cordatum, P. obtusum, Diplopsalis lenticula,Gonyaulax apiculata. From Cyanophycea Merismopedia punctata was recorded in noticeable numbers, and from green algae, Chaetoceros wighamii Brightwell (Rusakova 1995).
Shevchenko Bay of the Small Aral had a salinity of 29.5 ppt in 1992 and, as in the Tshche-Bas Bay of the Large Aral, the dominant species were brackish-water dinophycean algae (Exuviella cordata, Prorocentrum obtusum, Glenodinium lenticula f. lenticula,Peridinium trochoideum, Goniaulax spinifera,G. apiculata) and diatoms (Actinocyclus ehrenbergii). Various species of freshwater diatoms were also often encountered: e.g.,Cymbella lanceolata, C. prostrata, C. ventricosa; Gomphonema coronatum, G. ventricosum, G. olivaceum, G. truncatum var.capitatum; Tabellaria flocculosa, T. fenestrata, and also Diatoma tenue var.elongatum, Synedra ulna var. ulna,S. radians var. radians; Navicula cryptocephala var. cryptocephala were found in the desalinated area at the mouth of the Syr Darya. Blue-green and green algae were rare (Rusakova 1995).
Syr Darya delta of the Small Aral. These habitats had the greatest diversity with 128 recorded forms (Rusakova 1995): Cyanophycea (9), Chrysophycea (1) Bacillariophycea (66), Dinophycea (6), Chlorophyta (42), Charophyta (4). In relation to salinity, species numbers were: indifferent to salinity level (85), halophiles (2), mesohalobes (14), marine euryhaline (5), of unclear ecology (2). The more abundant forms included both freshwater and some halophilic forms. Cyanophyceae: Merismopedia tenuissima Lemmermann, Snowella lacustris (Chodat) Komárek et Hindák, Raphidiopsis setigera (Aptekarj) Eberly; Bacillariophycea:Tabellaria fenestrata (Lyngbye) Kützing, T. flocculosa (Roth) Kützing, Pantocsekiella kuetzingiana (Thwaites) K.T. Kiss et Ács, Fragilaria vaucheriae var. parvula (Kützing) Cleve-Euler, Nitzschia acicularis var.acicularis (Kützing) W. Smith; Chlorophyta: Scenedesmus quadricauda (Turpin) Brebisson, Tetradesmus lagerheimii M.J. Wynne et Guiry, Pseudopediastrum boryanum (Turpin) E. Hegewald,Monoraphidium griffithii (Berkeley) Komárková-Legnerová,Mucidosphaerium pulchellum (H.C. Wood) C. Bock, Proschold et Krienitz.Actinocyclus ehrenbergii was not among the dominant species (Rusakova 1995).
Hypersaline Large Aral. Here Sapozhnikov et al. (2009) and Zhitina (2011) recorded a total of 145 species of microalgae, the vast majority of which were not found in the early 1990s by Rusakova (1995). In these residual water bodies the largest number of species belonged to the Bacillariophycea (103), with Nitzschia,Tryblionella, Halamphora, Navicula and Amphora being most diverse. The remaining groups had few species such as Cyanophycea (12), Dinophycea (1) and Chlorophyta (11). Species diversity in the Eastern Large Aral (salinity 210 ppt) is approximately three times lower than in the less hypersaline Western Large Aral (salinity > 110 ppt).
Macrophytobenthos
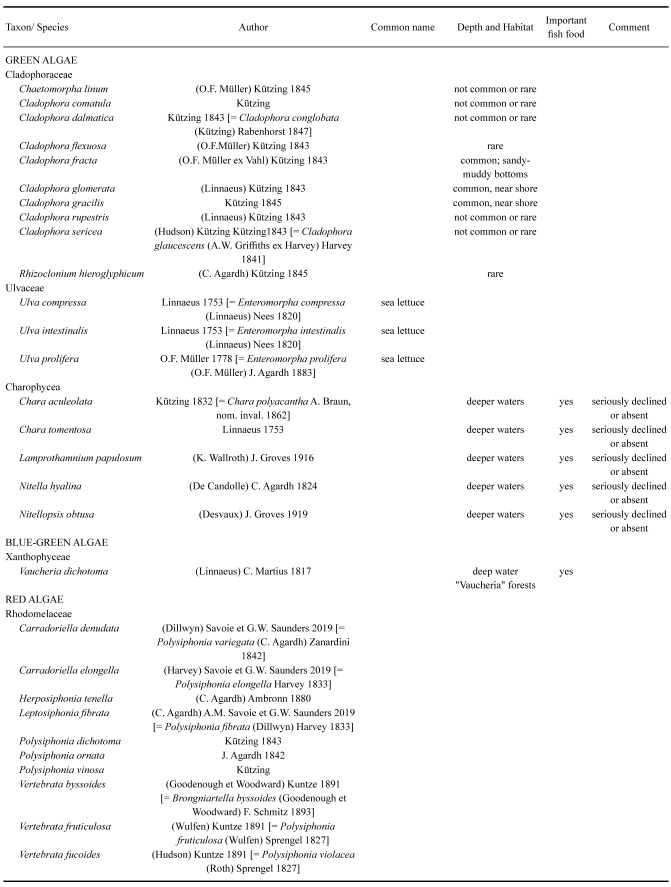
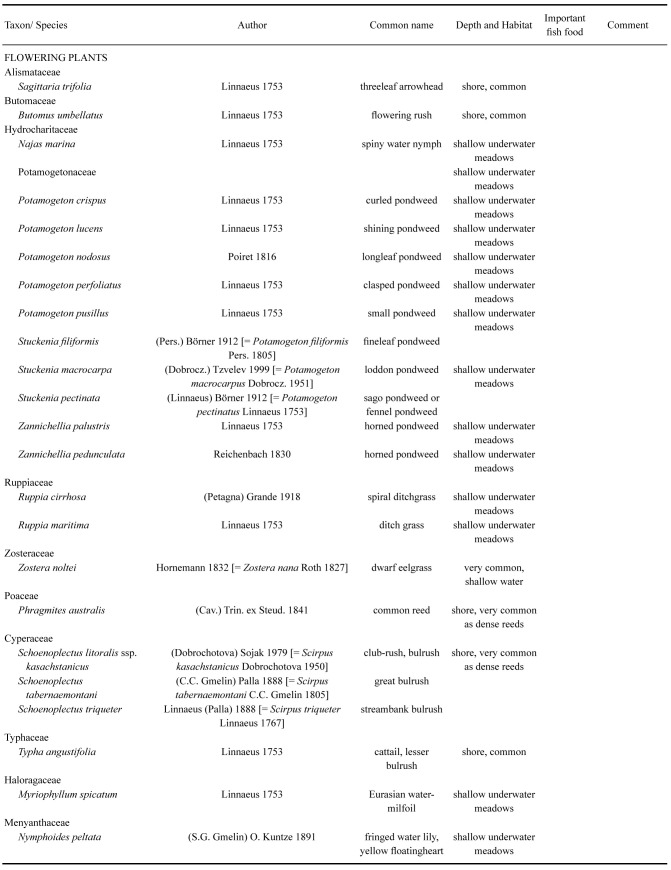
The original Aral Sea had a rather diverse flora of macrophytes. By 1960, it comprised 24 species of angiosperms, five species of charophytes and about 40 other species of macroalgae (Table 6). Many of these were important components of the diverse habitats and provided both food and shelter for several species of fish (Behning 1935; Bervald 1964; Dengina 1954 1959; Dobrokhotova 1971; Husainova 1960; Yablonskaya 1964; Zhakova 2013). Macroalgae of the Aral Sea were represented by green algae (Chlorophyta), Charophyta, yellow-green algae (Ochrophyta, Xanthophyceae) and red algae (Rhodophyta).
Table 6.
Macrophytobenthos of the original Aral Sea
Cladophora (Ulvophyceae): There were nine species of these filamentous green algae (Berg 1908; Behning 1935; Zhakova 1995 2013). Cladophora fracta preferred sandy-muddy soil, whil_e C. glomerata_ and C. gracilis preferred coastal areas. They are also fouling algae, forming dense thickets on ship hulls and piles, where they are favored by better water aeration. Several of the remaining species were either rare and some recorded only by a single author.
Ulva (Ulvophyceae) occurred with up to 12 species, including three species of Ulva (U. intestinalis L., U. compressa L., U. prolifera O.F. Müller), but it is now difficult to establish how widespread most of these forms were (Berg 1908; Zhakova 1995 2013). U. compressa is known only from the collections of Borshchov (Berg 1908), while U. intestinalis and U. prolifera were found by Zhakova (1995 2013).
Charophyceans were, according to Zhakova (2013), represented by six species from the genera Chara (2 spp.), Lamprothamnium (1 sp.) and Nitella (2 spp.). All of these are branching forms with leaf-like structures. Unfortunately, their systematic affiliations remain unclear, and this has since clouded what species were actually meant in the original accounts. These algae were found in the Aral Sea by Alenitsyn (1875) on silty soils at a depth of 11–22 m, where they formed dense extensive thickets. Berg (1908) also found such thickets at 18–23 m. At that time, these characeans were called Tolypella aralica Golenkin, which is a nomen nudum due to the fact that M.I. Golenkin never published its description (Gollerbach 1950). Despite this, in subsequent publications (Behning 1934 1935; Karpevich 1975; Aladin and Kotov 1989), the name_T. aralica_ was continuously applied to all charophytes from the Aral Sea (Zhakova 2013). The first information about the real species composition of Aral characeas was by Dengina (1959), who found two species, Chara aculeolata and Lamprothamnium papulosum.
Xanthophyceans (yellow-green algae) were not mentioned by Berg (1908), and they were first indicated by Behning (1935). The group is represented only by_Vaucheria dichotoma_ (L.).
Rhodophyta (red algae) remain an almost unexplored component of the Aral Sea flora. Borshchov (1865 1877) found 10 species of macrophytobenthic red algae (Berg 1908), all belonging to five genera of the Rhodomelaceae, including Polysiphonia (3 spp.), Vertebrata (3 spp.), Carradoriella (2 spp.), Herposiphonia (1 sp.) and Leptosiphonia (1 sp.). All these are small, thin, highly branched algae, usually attached to some kind of substrate. Vertebrata fucoides was the most widespread red alga in the Aral Sea (Behning 1935). In the 1990s, red algae were no longer found anywhere in the Aral Sea (Zhakova 2013) and they seem to have disappeared no later than the 1970s.
Angiosperms. The aquatic flora of flowering plants comprised 24 species (Zhakova 2013; Table 6). The majority belonged to the Alismatales, with the pondweeds (Potamogetonaceae) alone accounting for ten species. This is hardly surprising, since the Alismatales are known to host plants that can tolerate a range of salinities, such as_Zostera_ and Ruppia. The Poales were represented by five species including the cosmopolitan, Phragmites australis, three species of the cyperacean Schoenoplectus and the cattail Typha angustifolia. Present were also the spiked water-milfoil Myriophyllum spicatum (Haloragaceae) and fringed water lily Nymphoides peltata (Menyanthaceae). Since Zhakova (2013) there have been no studies on these plants so the present situation for the angiosperm flora remains unknown.
Microalgae and the food web
In the Aral Sea, the main source of biogenic nutrients came with the inflowing rivers, which had a relatively low content of salts, organic particles and colloids. The resulting nutrient poor waters of the Aral Sea therefore had and still have a paucity of planktonic microalgae. Moreover, most particulate matter carried by the rivers is sedimented near the deltas, and the overall effect is therefore very transparent waters that favor the development of benthic macrophytes (such as Chara) and benthic microalgae. Altogether, the nutrient poor waters reduced the primary production available to food webs of the Aral Sea (Karpevich 1975).
Phytoplankton is devoured by planktonic crustaceans, principally copepods and cladocerans, but the paucity of these algae explains why there are no fish from the open water that depend entirely upon zooplankton. Both species diversity (128 spp.) and biomass was highest in the freshened area (salinity 5–7 ppt) in front of the Syr Darya delta in the Small Aral Sea. This area was and is still favorable for freshwater and fresh-brackish water species (Rusakova 1995) due to sufficient warming, a relatively low salinity and a somewhat higher amount of biogenic elements than in the sea at large. The species diversity of phytoplankton in the Tshche-Bas (Large Aral) and the Shevchenko and Butakov Bays (Small Aral) were much lower than in the freshened area of the sea, primarily due to the almost complete loss of green algae.
Benthic microalgae. The paucity of plankton algae meant that microalgae on surfaces were an important food source. In the bottom mud invertebrates fed largely on diatoms coating the silt particles, although detritus also formed part of their diet (Behning 1935). Important components in this fauna were chironomid larvae, mollusks, the now extinct amphipod, nematodes and oligochaetes all of which were again dietary components of fish. It is likely that various prokaryotes may also cover macrophytes in the near-shore reed beds, where they could be an important food item for small, grazing animals (Mossin 1988), but there is no data on these organisms.
Macroflora and habitats
In the original Aral Sea the range in depth and salinity and other variable conditions enabled the existence of a variety of plant communities in deeper waters, along the shores and in the delta regions. Several macrophytes occurring commonly in deeper waters,e.g., Chara spp. and Vaucheria dichotoma were at various periods important as food for fish. These were mainly roach but also white-eyed, bream, aral barbel, shemaya and sabrefish (Behning 1935; Pankratova 1935). As for animals, most changes to vegetation can clearly be attributed to the increasing salinization after 1960, but some occurring prior to that time still warrants a satisfactory explanation.
In deeper waters the benthic macrophytes could form dense vegetative mats as deep as 30 m (Table 6), and they greatly increased oxygen levels of the bottom layers. At the start of the 20th century characean algae formed extensive thickets at a 18–23 m depth (Alenitsyn 1875; Berg 1908). Yet, by the 1930s these characean were no longer recorded in deeper waters, but mainly on black silt in more or less isolated bays (Behning 1935). It is not known exactly when and why the deep-water characeans disappeared. They are known to prefer clear waters with a low content of nutrient salts and organic matter, and it is entirely possible that human caused changes to the waters may have accelerated their demise. By the 1950s the characeans had definitely been replaced by the yellow-green alga Vaucheria dichotoma, which formed dense aggregations called “Vaucheria forests” on gray silts. In the Small Aral Sea, these tickets occurred down to 26 m and were important food items for several fish species (Zhakova 2013).
In shallower waters and before the modern regression crisis, small but constant fluctuations in salinity in the bays and along the coasts were favorable for the existence of both freshwater and brackish water vegetation, resulting in a diversity of plant communities forming belts of hydrophytes and helophytes.
Helophytes are perennial marsh plants with buds overwintering underwater. In the sea, they formed clumps and border thickets located in a continuous or discontinuous strip from 1 to 100 m wide. They were more significant on the south and east coasts than on the north and west. Thickets of Phragmites australis dominated everywhere. The maximum height of these reed beds reached 4.5 m, with a density of up to 300 plants/m2 and they produced 2.8 million tons of organic matter per year. In the northern part of the Aral Sea, behind the reed zone, there was often a band of Schoenoplectus litoralis kasachstanicus (club rush), forming thickets up to 4 m high at depths from 1.5 to 3.5 m and a density of up to 25 plants/m2 (Behning 1935; Bervald 1964; Dobrokhotova 1971; Yablonskaya 1964; Zhakova 2013). Other helophytes (Butomus, Sagittaria, Typha) did not form such significant thickets.
Hydrophyte communities were more abundant in the northern part of the Aral Sea as various associations formed vast underwater meadows, and comprised species of_Potamogeton_, Zannichellia, Ruppia,Zostera, Myriophyllum, Najas and_Nymphoides_ (Table 6). In the seaward part and in open bays dwarf eelgrass (Zostera noltei) dominated on sandy bottoms at depths of 3–11 m, where it formed continuous or discontinuous thickets. The productivity of the_Z. noltei_ communities was so high that the layer of leaves thrown ashore reached 0.8–1 m in thickness and 2–3 m in width along a considerable length of the coastal strip. This resembles conditions that existed in Z. marina communities in many areas of Western Europe, before the arrival of the “Zostera wasting disease” that affected this species dramatically. Interestingly, this disease seems not to have affected Z. noltei although this slime mold has been identified in its tissues (Vergeer and Hartog 1991). This apparent “immunity” may well have spared the Aral Sea communities of an early and major ecological change. According to Zhakova (2013) Z. marina is not present in the Aral Sea.
In Bolshoy Sary-Chaganak bays, the macrophytes consisted of mostly non-Alismatales species forming small associations of one to three species: 1.Zostera noltei + Ruppia cirrhoza +Chaetomorpha linum (salinity 21–26 ppt); 2. Ruppia cirrhoza (salinity 21–26 ppt); 3. Chaetomorpha linum + Cladophora glomerata + Cl. fracta (salinity 20–30 ppt) (Zhakova 1995).
Salinized, closed bays were dominated by charophytes (Bervald 1964; Dobrokhotova 1971; Yablonskaya 1964).
Fauna disturbances by introduced species
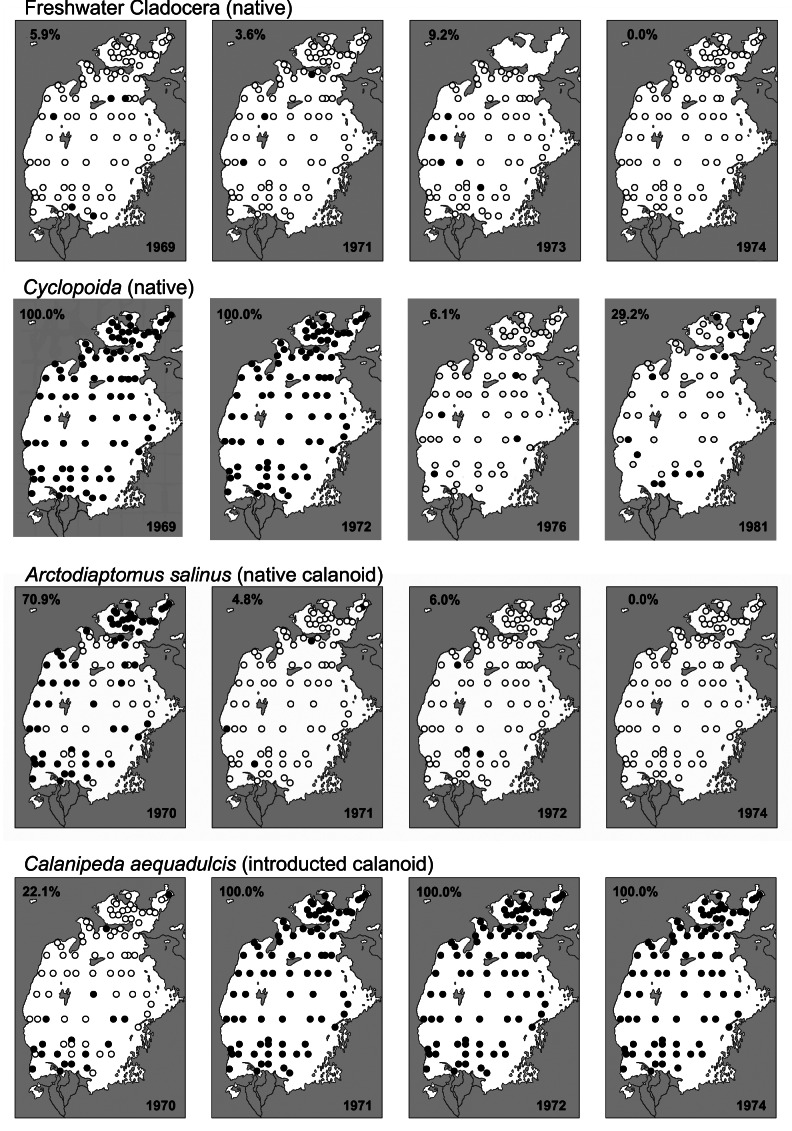
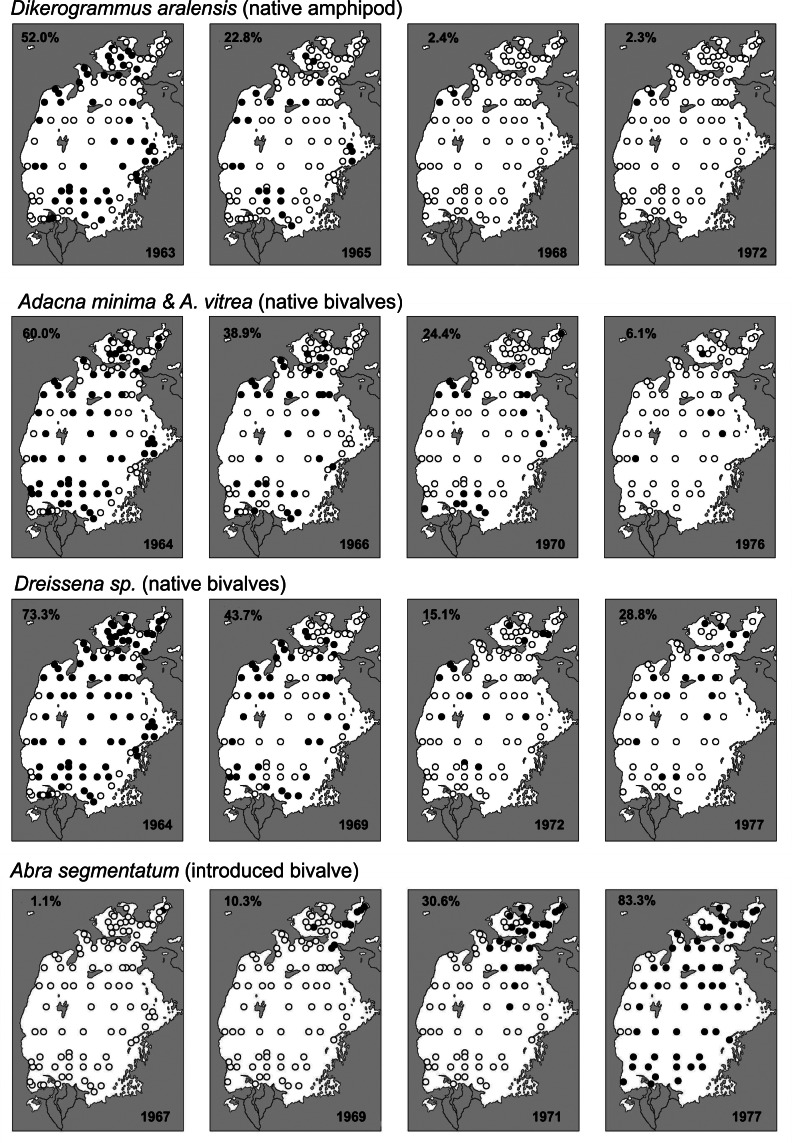
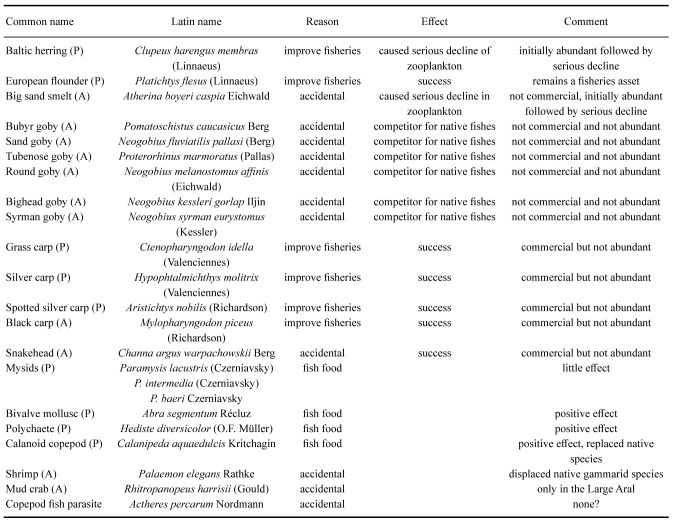
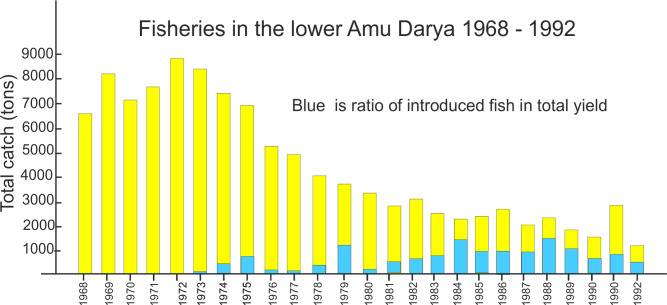
Both planned and accidental introductions of animal species occurred during the latter half of the 20th century (Table 7, Aladin et al. 2019). The planned introductions were intended to increase commercial fish stock and involved both non-native fishes and invertebrates considered suitable as fish food. These efforts were unrelated to the increasing salinization crisis (Figs. 6–8). The euryhaline polychaete worm Hediste diversicolor was introduced in 1960–1961. In 1973–1974 it had spread around the Aral Sea and become a valuable food for the fish. The euryhaline bivalve Abra segmentum was introduced in 1960–1963, and by the mid 1970s had spread to the whole Aral Sea and become a valuable food for the fish (Fig. 8). In contrast, almost all introductions of fish and crustaceans had either negative effects or no effects at all on the fisheries. Accidental introduction of the shrimp Palaemon elegans led to displacement of the native gammarid Dikerogrammus aralensis (Fig. 4). The planned introduction of the euryhaline and productive copepod Calanipeda aquaedulcis provided better food for the fish. Unfortunately, it displaced the already declining native species Arctodiaptomus salinus (Figs. 4, 7). Three species of mysids were also introduced, but only one, P. intermedia, became established and abundant in non-salinized areas (Plotnikov et al. 2021b). The planned introductions of fish were mostly unsuccessful or had catastrophic effects on the ecosystem (Ermakhanov et al. 2012). In the 1950s, attempts to introduce commercially valuable mullets (2 species), resulted in accidental introductions of the Caspian atherine (Atherina boyeri caspia) and several species of gobies (Pomatoschistus caucasicus, Proterorhinus marmoratus,Neogobius fluviatilis pallasi, N. melanostomus affinis, N. kessleri gorlap, N. syrman eurystomus). Simultaneously, Baltic herring (Clupea harengus membras) were also introduced. The mullets did not become established. The herrings and young atherine are pure planktivores and not typical of Aral Sea species. Their voracious feeding resulted in a dramatic decline in planktonic crustaceans, which again caused serious starvation of the fish. Thus the abundances of herring and atherine subsequently decreased to very low levels. In 1960–1961, there were introductions of introduced commercial fishes from the Far East (Ermakhanov et al. 2012; Plotnikov et al. 2016): the herbivorous grass carp (Ctenopharyngodon idella); fish consuming phytoplankton, zooplankton and detritus such as silver carp (Hypophthalmichthys molitrix) and bighead carp (Aristichthys nobilis) and also the predatory snakehead (Channa argus warpachowskii). They are now numerous, occurring in the Aral near the mouth of the rivers. The only real success for fisheries was the introduction of the European flounder, Platichthys flesus from 1979 to 1987. This took place under scientific and practical guidance from experienced Danish fishermen (Plotnikov et al. 2021b), and the result was that these bottom foragers became and remain a permanent asset for the fisheries in the Aral Sea.
Fig. 6.
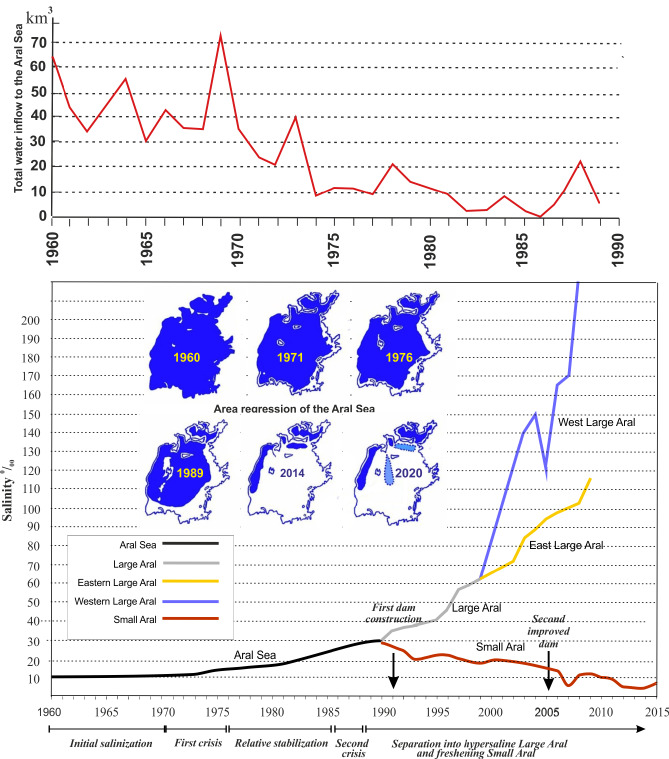
Salinity and inflow of water for the Aral Sea. A, Total inflow to the Aral Sea during the regression and caused by diversion for irrigation purposes. B, The resulting increase in salinity; after 1990 the waters of the Large and Small Aral were separated and evolved independently of each other; the inserted maps show the decrease in area. After 2000 also the eastern and western parts of the Large Aral became separate water bodies, but both rapidly hypersalinizing. The construction of the dams that kept water in the Small Aral, had almost immediate effects. On the axis is indicated key events for the fauna in the system; further details in text. Modified and extended with new data from Plotnikov et al. (2021b).
Fig. 7.
Zooplankton recorded at fixed stations during the crisis. The percentages indicate the number of fixed sampling stations where the taxon was recorded. Native cladocerans, cyclopoids and calanoids all declined. The introduced calanoid_Calanipeda aquaedulcis_ initially spread throughout the sea. Detailed methodology explained in Plotnikov et al. (2021b).
Fig. 8.
Zoobenthos collected at fixed stations during the crisis. The percentages indicate the number of fixed sampling stations where the taxon was recorded. Detailed methodology explained in Plotnikov et al. (2021b).
Table 7.
Planned (P) and accidental (A) species introductions to the Aral Sea. Planned introductions were all performed to improve fisheries, but only the European Flounder was a success. All introduced species, except Paramysis baeri, became established, but some eventually declined to very low abundance. Further information in text and in Plotnikov et al. (2021b)
DISCUSSION
Regression and salinization
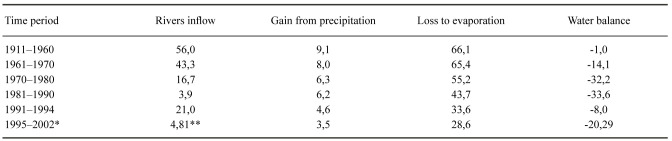
The modern regression crisis commenced at the middle of the 20th century due to the accelerated diversion of water in the tributary rivers for human purposes, mainly irrigation (Figs. 7, 9). Historically, water has long been used from the Amu Darya and Syr Darya to sustain agriculture, and this enabled the sprawling cultures located here in the Middle Ages (See e.g., Frye 2011). Despite this, the salinity of the Aral Sea remained rather stable, inasmuch as these advanced cultures were able to economize water usage (see below). From around 1960, water was increasingly used for irrigating new water demanding crops, principally cotton, and also to sustain the increasing population. The reduced inflow resulted in an increasingly negative water balance, whence the area of the Aral Sea started to shrink and, accordingly, the salinity to increase (Figs. 2, 6; Table 8).
Fig. 9.
The Aral Sea regression crisis. A, The desert left by the vanished sea is slowly being populated by drought and salt resistant plants. B, The first, primitive dam over the Berg Strait. C, Local fishermen look from the north at the final Kokaral Dam over the Berg Strait. D–E, ESA Spot satellite images of the Small Aral Sea soon after the dam construction and showing the increase in area already during the first year. F, Commercial fishing from small boats in the reconstituted Small Aral Sea.
Table 8.
Water Balance of the Aral Sea (km3/year). Data from Agency of IFAS (Agency of IFAS for implementation of the Aral Sea basin)
Effects on invertebrates
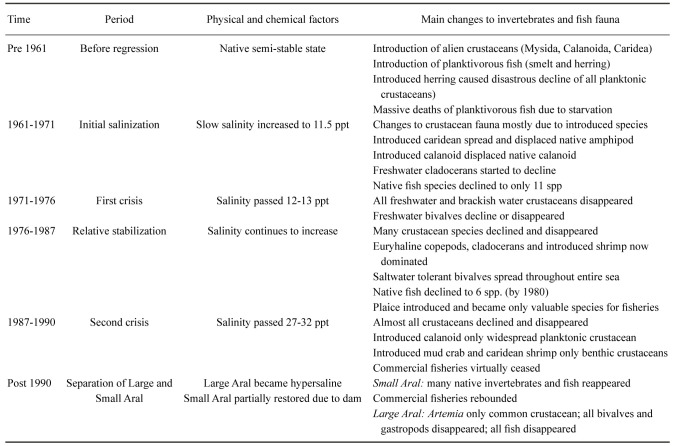
During the first years of area regression, the biodiversity was largely unaffected by the salinity increase itself, although disturbances were caused by the planned or accidental introduction of alien species. Over time the various types of organisms met their upper salinity tolerance and decreased to minimal levels or vanished completely (Table 7; Figs. 7, 8). Following Plotnikov et al. (2021b) we divide the events into three crises. The (1) first crisis occurred from 1971–1976, when salinity reached levels (12–13 ppt) that caused true freshwater crustaceans to disappear. Sharp declines were also seen for the bivalves, where Adacna and Dreissena became less common and_Cerastoderma_ sp. A disappeared entirely by 1976 (Table 7, Fig. 8). Following the first crisis, conditions remained relatively stable for a time, allowing both brackish water and euryhaline species to thrive or even to increase. Thus, the saltwater tolerant bivalves C. glaucum and A. segmentum spread to the entire sea (Figs. 4, 8). The halophilic gastropod Ecrobia grimmi also increased in abundance (Andreeva 1989). But irrespective of this, the entire molluscan fauna eventually declined due to the increasing salinization. The (2) second crisis occurred from 1987–1990, when salinity reached 27–32 ppt, causing a sharp decline in species. For crustaceans, most native species, including cladocerans, completely disappeared. Only the introduced calanoid copepod Calanipeda aquaedulcis, remained in the plankton (Table 7).
The (3) final crisis happened around 1990. At this time, the original continuous sea had become separated into a southern Large Aral and a northern Small Aral. The large Aral became several hypersaline bodies of water, a western deeper part and a more shallow eastern part. All fish and euryhaline invertebrates have vanished in these remnants of the Large Aral and only hypersaline tolerant metazoan species survived. The brine shrimp_Artemia_ began to spread in these waters. The bivalve_Cerastoderma glaucum_ disappeared by 2001 and Abra segmentum by 2004. By the 2000s the salt water tolerant gastropod Ecrobia grimmi also vanished in the Large Aral (Aladin and Plotnikov 2008; Plotnikov 2013). At present, the water bodies of the original Large Aral are steadily increasing in salinity, and it can be predicted that they will ultimately become like the Dead Sea with no metazoan life at all (Oren 2018; Plotnikov et al. 2021b).
Effect on ichthyofauna
Aside from the effects of species introductions, the successive extirpation of fish species was almost entirely due to the increasing salinity (Ermakhanov et al. 2012 2013). There is no historical data on standing fish stock but figure 10 shows a clear correlation between the decline in commercially caught fish and increasing salinity. Simultaneously, the number of fish species decreased from the original 22 to only the species of introduced flounder by 1990. Since almost all native fish had a wide diversity in their diet, they were minimally affected by changes in the available food species, just shifting to other food assets. This lack of specialized diet was also crucial to their survival in localized habitats, and part of why they could eventually repopulate the partially restored Small Aral Sea (see below). The decline in fish stock and species diversity was in some part due to their reproductive behaviour. For species spawning in freshened parts of the sea, the fry were much less salinity tolerant than the adult fish. The increasing salinity in the general sea may also have negatively affected the maturation of reproductive products in the adults, although this issue is still debatable.
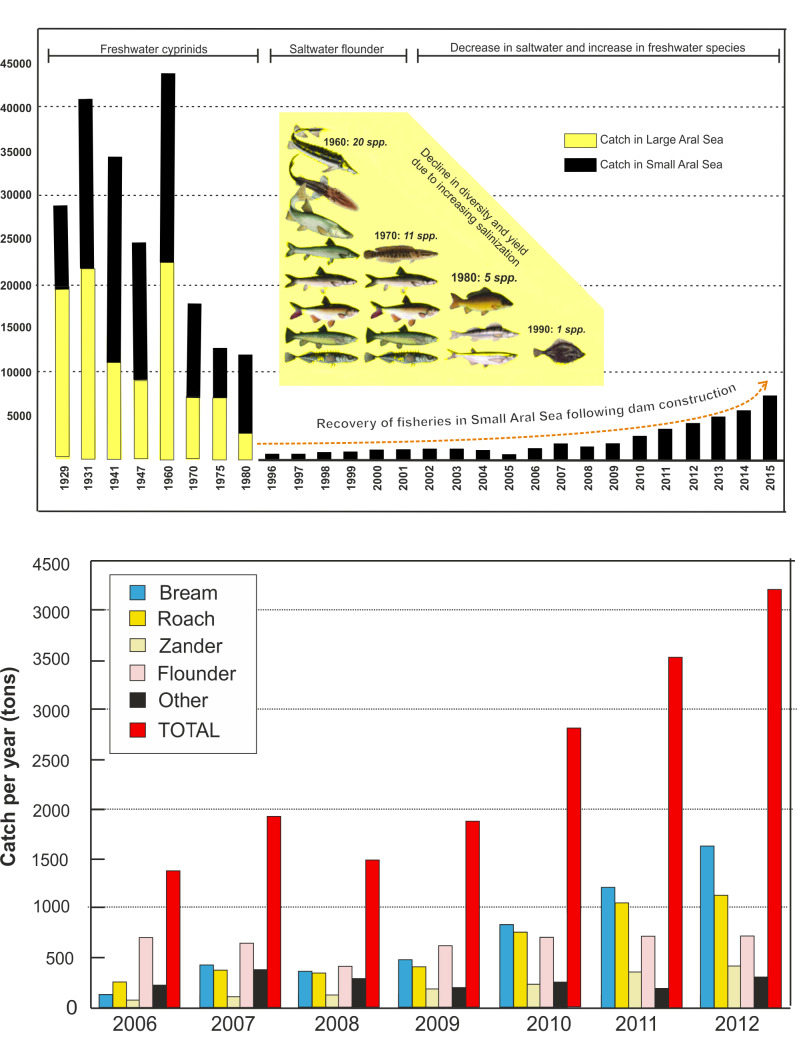
Fig. 10.
A, Commercial fisheries yield for the Aral Sea system. For early years only intermittent data are available. The yield declined to virtually nil during the regression crisis after 1960, but then recovered in the partially restored Small Aral Sea. The inserted figure shows the decline in fish biodiversity. B, Yield per species of commercial fisheries in the restored Small Aral Sea. Data after 2012 are not completely available.
Effect on birds
It is obvious that the disastrous reduction in area and coastline of the Aral Sea must have had a significant effect on the birds associated with these habitats, but we are unaware of detailed studies. Migratory birds often depend on very specific resting grounds, and if these are damaged or disappear, it may have catastrophic effects because no alternative sites may exist (Reneerkens et al. 2005; Pennisi 2015). However, it can be hoped that the reconstituted Small Aral and the areas around the river deltas will still furnish enough habitats for the birds to avoid this kind of catastrophy. Just like mammalian wildlife, birds are an important asset for ecotourism, which may benefit the local economy and assist efforts at habitat conservation, although increased tourist flow by itself also poses a threat (Kumar and Sheryzasdanova 2021). For the flamingo, the present availability of brine shrimp may be an asset, but this food source is threatened by the increasing hypersalination. Fortunately, there are smaller saline lakes to the north-east of the Aral Sea (Lake Shalkar and Lake Tengiz) that offer alternative grounds. Flamingos are now even flocking in the small Maly Taldylkol lake in the center of Kazakhstan’s capital Astana, formerly called Nur Sultan (UN News 2021). Nevertheless, although some of these lakes are part of nature reservations, these localities are also threatened by human activities.
Effects on macrophytobenthos
The regression salinization also led to a catastro-phic reduction in the biodiversity of macrophytes and the demise of most biocenoses just as was the case for the fauna. Of the flowering plants, the first extirpations due to salinization were the freshwater and freshwater-brackish-water hydrophytes. Within a few years, freshwater pondweeds (Potamogeton) disappeared, followed by the more resistant_Myriophyllum spicatum_ and comb pondweed Stuckenia pectinata. By the end of the 1970s, a few euryhaline species became the dominant species. During this time, the cover of reed thickets had become reduced to half their former coverage. They were at first restricted to a vast near shore zone out of the water but then disappeared completely in the 1980s (Zhakova 2013). Studies performed on the salinity gradient of the Karabayli archipelago (Dengina 1959) showed that reeds developed normally at a salinity of up to 18.5‰ but died at 24‰. Reed thickets of_Schoenoplectus litoralis kasachstanicus_ disappeared at 16‰ salinity. New and rapidly salinizing shallow-water biotopes were rapidly overgrown with the halophilic annuals such as Zanichellia spp., Ruppia spp. and the characean Lamprothamnium papulosum. With a further increase in salinity above 25–26‰, these species also disappeared (Dengina 1954 1959; Husainova 1960). By the end of the 1980s there only remained Ruppia spp.,Cladophora fracta, C. glomerata, Chaetomorpha linum, Rhizoclonium hieroglyphicum, Ulva intestinalis, and U. prolifera, all of which are able to withstand high salinity (Zhakova 2013).
In the Small Aral, where salinity began to decrease in the late 1990s, the predominating macroalgae were Chaetomorpha linum, Cladophora glomerata, C. fracta. Macrophyte communities were now formed by four species of flowering plants: Phragmites australis, Ruppia cirrhosa, R. maritima, Zostera noltei and the charophytes Lamprothamnium papulosum and Chara aculeolata. R. cirrhosa communities dominated in sheltered bays on silty bottoms at depths of 0.7–1.2 m. On sandy bottoms at depths of 1.2–4.5 m, the macrophyte cover was formed by Z. noltei. Every surface that could be attached to was overgrown with green algae (Zhakova 1995; Orlova and Rusakova 1995), probably indicating a fairly high level of mineral nutrients. The communities of_L. papulosum_ were very rare. Water thickets of reeds began to form near the Syr Darya delta. At present, salinity of the Small Aral continues to decrease gradually (Fig. 6), and the water body is populated by widespread halophilic, cosmopolitan and extremely polymorphic species of hydrophytes and helophytes penetrating from other continental brackish water bodies of the general Aral Sea region.
By the beginning of the 2000s, in the residual and hypersaline remnants of the Large Aral, macrophytes were reduced to only Cladophora fracta, C. glomerata, Vaucheria dichotoma and some sterile specimens of_Ruppia_ sp. (Sapozhnikov et al. 2009; Zavialov et al. 2003; Zhakova 2013). In the western parts, the most abundant species was C. fracta, found in abundance in coastal areas at depths of up to 1–1.5 m, sometimes covering up to 80% of the bottom. C. glomerata occurred infrequently in the form of individual clumps. The formerly widespread Vaucheria dichotoma was found in small numbers and only at depths of 0–3 cm on layers of hard clay. The more tolerant_C. glomerata_ was the only species found in the more salinized Eastern Large Aral (Sapozhnikov et al. 2009). With the ever-increasing salinization, the macroflora will expectedly become even further impoverished and eventually vanish altogether.
The new desert and terrestrial habitats
Kazakhstan in general has very valuable and biodiverse flora and fauna, much of which is endangered or even redlisted (CBD 2018). The majority of this diversity are terrestrial organisms, some of which were also affected by the Aral Sea crisis. The regression left a vast, dry area now covered with salty sands (Issayeva et al. 2011). Because of the lack of vegetation as much as 1.5–2 cm of soil is blown away every year in a process called deflation. This new desert, called the Aralkum, is gradually being populated by dry-tolerant vegetation, such as species of Chenopodiaceae, and is thus slowly being converted into a dry steppe habitat (Fig. 9). These events have been monitored over more than 25 years, and the Aralkum can therefore be used as an illustrative and informative example of what could happen in other arid areas of the world such as the southern border of the Sahara and the Lake Tchad or arid areas in the south-western United States (Wucherer and Breckle 2001). Fortunately, studies are now being conducted on how to rehabilitate the dried out sea bottom by planting new vegetation (Kim et al. 2020). As an example, the ongoing planting of forests is reducing wind speed and thus the deflation process (Bakirov et al. 2020).
The regression crisis also has very significant effects on terrestrial areas never covered by the Aral Sea. Formerly, the Aral Sea acted as a buffer that dampened temperature oscillations, much like areas adjacent to the oceans. During the pre-desiccation period analysis of long term trends indicated a negative air temperature trend (Khan et al. 2004). Following the regression, summers have become hotter, and violent weather events with stronger winds have occurred with increasing frequency (Deliry et al. 2020; Markovic et al. 2014; Lioubimtseva 2014). These dynamics have been exacerbated by the ongoing global warming. These events must affect the terrestrial flora and fauna, although to what extent is far from clear. Global warming may also affect both aquatic and terrestrial systems in several ways. Nevertheless, accurate studies, including satellite monitoring, show that vegetation cover has generally increased, especially in Kazakhstan, with only Kyrgyzstan having a consistent loss (Liu and Chen 2021). Yet, the true pattern may be more complex, as the net values may obscure the fact that dry tolerant plants are becoming more common in areas, while less tolerant species are declining.
The area around the Aral Sea was and is very biodiverse hosting 638 (Agency of IFAS 2022) species of flowering plants and a rich fauna of vertebrate animals. Many of these steppe or desert animals are now endangered or even at the verge of extinction. 12 species of mammals, 26 species of birds and 11 plant species have almost disappeared and several have gone extinct. There are many natural parks and protected areas in the general Aral Sea region, but they face difficult problems both financially and politically. A positive example is the Altyn-Emel national park (459,627 ha), created in the Ili River Valley in Kazakhstan in 1996. In spite of developed ecotourism and trophy hunting, the ungulate populations in the park are increasing very significantly. Thus, the kulan (Equus hemionus kulan) went extinct in Kazakhstan in the 1930s, but was re-introduced by the 1950s, apparently with only 32 individuals. The population size increased steadily and by 2000 it amounted to 500–600 individuals (data from the World Wildlife Foundation). Yet, while the kulan and many animals prosper in the protected parks, they are only present in a tiny fraction of their former range in Kazakhstan, and the same is true for many other animal species. Hopefully, increased ecotourism carried out under sustainable conditions may in the future contribute to the conservation efforts in the Aral Sea region (Saidmamatov et al. 2020).
Human health effects
Aside from affecting the Aral Sea biodiversity and fisheries, the regression crisis also directly affected the health of the population in several and serious ways (e.g., Whish-Wilson 2002; Ataniyazova 2003; Wâhler and Dietrichs 2017; Anchita et al. 2021). The general health status in the Aral Sea area declined significantly during the crisis, but the direct reasons for this are not always clear (Anchita et al. 2021). The salt pans resulting from the regressions allow for salt dust to be was carried into the air (Chen et al. 2022) and this has been suspected to cause health problems. Similar threats may now also be imminent in the area around the Great Salt Lake (Kintisch 2022). In the Aral Sea area, worsening weather conditions exacerbate the situation by increasing the frequency of dust storms. Adding to the severity of these storms is the fact that the soil is badly contaminated with both chlorinated pesticides from agriculture and likely with waste from a chemical warfare plant formerly located on Vozrozhdeniya Island. In Turkmenistan alone, 50% of all reported illnesses in children are related to respiratory system difficulties. In Kazakhstan, infant mortality increased 50% from 1985 to 2005. In 1990, it was 60–110/1000 compared to only 48/100 in Uzbekistan and 24/1000 in USSR Russia (Wâhler and Dietrichs 2017; Wikipedia 2022). However, from 2009 to 2019 infant mortality in Kazakhstan decreased steadily from 25/100 to 9/100, something that may be correlated with an economic recovery in the country that is also reflected in a slowly decreasing level of unemployment. According to data in Anchita et al. (2021) there is no clear evidence that serious health problems are due to the increasing frequency of dust storms. More likely, the entire population in the area has for 40–50 years been constantly exposed to chlorinated pesticides that ended up in both soil and drinking water and simultaneously in animal and plants used for food, causing a multitude of chronic health problems, including cancer (e.g., Wâhler and Dietrichs 2017; Ataniyazova 2003). We are not aware of any studies concerning the extent to which the air and soil pollution have also affected the rich wildlife in the Aral Sea area.
Unfortunately, health problems comparable to those experienced at the Aral Sea may now be threatening the population around the Great Salt Lake, including airborne dust containing arsenic and metals derived from industry, wastewater and other sources (Kintisch 2022).
Restoration of the Small Aral
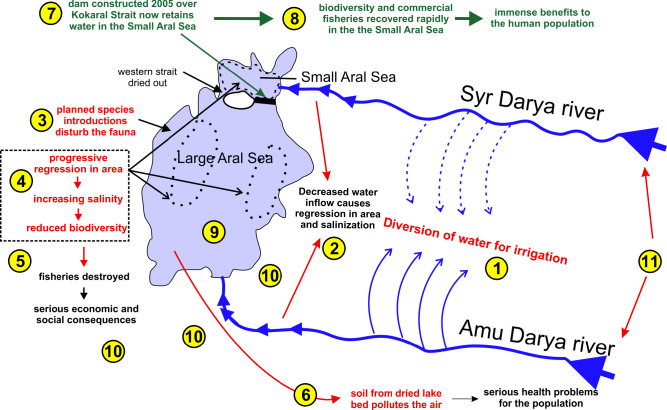
Around 1990 the Large Aral Sea had become a system of hypersaline water bodies. It consisted principally of the deeper Western Large Aral and the shallower Eastern Large Aral and they both only irregularly received any water from the southern Amu Darya (Fig. 2). In contrast, the Small Aral Sea constantly received water from the Syr Darya, but the outflow, now solely through the Berg Strait, did not reach any of the residual Large Aral parts but instead just spread and evaporated on the salt pans. Following the demise of the USSR and stimulated by the ongoing Glasnost, it became possible by local means to build a dam across the Berg Strait to contain the waters of the Small Aral Sea (Micklin 2016). Within a few years, hydrodynamic forces damaged this primitive first dam but the beneficial effects were so dramatic that funds were raised for constructing the present Kokaral Dam in 2005 (partially by means from the World Bank (Micklin 2016)). As a result, the area of the Small Aral Sea started to increase and the salinity started to decrease (Figs. 6, 9). In 2008, only three years after construction, the water level had risen 12 m above that of 2003. The Small Aral is now a stable brackish water body with a surface area of 3,230 km2, and many of the original fish and invertebrate species have re-emerged. No planned species introductions took place, so the fauna must have come from refugia in the freshened parts of the river and delta regions of the Syr Darya. Some crustaceans may also have survived as resting eggs in the dried out parts (Plotnikov et al. 2021b).
Present Fauna
Small Aral. In the plankton are now found four copepods (Phyllodiaptomus blanci, Cyclops vicinus,Mesocyclops leuckarti, Megacyclops viridis) and seven cladocerans (Bosmina longirostris, Chydorus sphaericus,Diaphanosoma brachyurum, Ceriodaphnia reticulata,Evadne anonyx, Podonevadne camptonyx, P. angusta). In the benthos are found the mysid Paramysis intermedia, the caridean Palaemon elegans, three bivalves (Dreissena polymorpha aralensis, Cerastoderma glaucum,Abra segmentum) and two gastropods (Theodoxus pallasi,Ecrobia grimmi). The introduced polychaete worm Hediste diversicolor is also in the benthos. The introduced bivalve A segmentum dominates the benthos, but compared to the first half of the 1990s (Filippov 1995) the abundance of all molluscs remains depressed. In the case of Cerastoderma glaucum and Ecrobia grimmi, this can be explained by a decrease in salinity to levels unfavorable for them (Plotnikov et al. 2016). Another reason for the decline of molluscs, primarily A. segmentum, was increased predation by fish following the rapid recovery of the ichthyofauna (Ermakhanov et al. 2012). 16 of the original ichthyofauna remain, plus the introduced flounder. The sturgeon (Acipenser nudiventris) and the Aral salmon (Salmo trutta aralensis) have gone extinct.
Large Aral Sea. No fish or molluscs survived in the Large Aral. Few crustacean species either survived the crisis or appeared as new in the hypersaline bodies. The brine shrimp Artemia appeared in 1998, but was previously found only in small and isolated bodies of hypersaline water adjacent to the sea. In 2002, larvae of the chironomid Baeotendipes noctivaga were found (Mokievsky and Miljutina 2011). The copepod Apocyclops dengizicus appeared in 2004, the ostracod Eucypris mareotica in 2005, and the copepod Nitocra lacustris survived from the initial fauna. Sampling eggs of the brine shrimp has some economic importance, and this species is also a new and valuable food source for birds such as the flamingo. Nevertheless, all these invertebrates face eventual extinction due to the constantly increasing salinity in the Large Aral waters (Plotnikov et al. 2021b).
Fish and fisheries
Prior to 1960 the total yield of fish from the Aral Sea was significant, although variable (Fig. 10). It depended almost entirely on native cyprinid species. The yield constituted only about 5% of the total catch in the former USSR, but in terms of economic value, it came in second after that in the Caspian Sea. Furthermore, Aral Sea fisheries were an important source of trade and contributed the majority of the protein consumed by the local population (Ermakhanov et al. 2012 2013; Plotnikov et al. 2021b).
With the onset of the area regression, increasing salinization caused a rapid decline in both yield and diversity of fish species (Fig. 10). The total catch declined from approximately 43,000 tons in 1960 to approximately only 12,000 tons in 1980 and by the late 1990s was almost insignificant, with the introduced flounder being the only asset. By 2003, before the partial recovery of the Small Aral Sea, some 40,000–60,000 fishermen in Kazakhstan had lost their jobs. During the crisis period, the relative proportion caught in the small Aral increased from about 50–100%, since following hypersalinization of the Large Aral Sea no fish remained after 1990 (Fig. 10).
The recovery of the Small Aral, due to dam construction, had an almost immediate effect on fisheries that gradually increased. The commercial fishery is again thriving and an important economic asset in the area (Figs. 9, 10). Although we lack data for the most recent years, the trend indicates that the catch in the Small Aral will approach that seen before the onset of the crisis. Fisheries in the Aral Sea system never returned to industrial levels, but small-scale fishing continues in the Small Aral, using either small boats or fishing from the ice during winter (Fig. 9). This suggests that overutilization of the stock may not become a serious problem.
Presently, fisheries in the Small Aral depend largely on four species, with the economically valuable sander being an important asset (Fig. 10). Unfortunately, we have been unable to retrieve data on the economic value of both the entire trade and the individual species. Similarly, it remains obscure how much fish is sold and consumed locally and how much may be exported out of Kazakhstan to the benefit of the national economy.
Fisheries were and continue to be also important in the two tributary rivers, but yields became seriously diminished in the Lower Amu Darya during the crisis period (Pavlovskaya and Zholdasova 1991; Pavlovskaya 2022). The total catch was around 8000 tons/year until the mid-1970s, when this was more than half the catch of the Aral Sea. By 1992, the catch declined to just 13% of the 1972 level (Fig. 11). Native fish that migrated up the river for reproduction decreased in numbers as the Aral Sea salinized and decreased in area. Pollution and loss of fry that were lost in irrigation canals also contributed to the decline. Introduced fish species, mainly from the Far East, therefore became an increasing part of the otherwise decreasing total landings. However, these freshwater species depend on the existence of lakes and ponds associated with rivers, which became increasingly saline due to run off from the irrigated fields. Pesticides, herbicides and other types of pollution also present a looming problem for the use of fish. In the nearby Lake Sarygamysh, the fish are now so contaminated that they are unsuitable for human consumption. Fisheries in the lower Amu Darya can only recover if the river is allowed to flow into a reconstituted Large Aral Sea. At present, water flow into the vanished Large Aral is intermittent and the waters are mostly hypersaline and without any fish at all.
Fig. 11.
Total fish catch in the lower Amu Darya. In 1992 the landings had decreased to 13% of the amount in 1972. The blue part of columns is the ratio of introduced fishes in the total landings. Catches decreased due to the Aral Sea Crisis. (data from Pavlowskya 2022).
Recent hydrological changes
As a result of the desiccation, the original southern part of the Large Aral Sea, split into several mostly isolated water bodies: the western and eastern part and the now isolated Tshche-Bas Bay. Each separated region experienced different hydrological developments and hence presented very different ecological conditions for aquatic life (Izhitskiy et al. 2016; Andrulionis et al. 2022). The restored Small Aral has remained relatively stable, with conditions resembling the original brackish water lake. Yet an increase in sulfates has recently been detected that may be the result of run off from the Syr Darya. The remnants of the original Large Aral Sea are now hypersaline. In the western part, salinity surpassed 140 ppt and is still increasing (Andrulionis et al. 2021). Due to precipitation of carbonate and gypsum, the ionic composition has changed with divalent ions (calcium and sulfate) decreasing relative to chloride (Zavialov et al. 2009a). The situation is complicated by the occasional presence of a channel, probably formed by erosion, that enables some exchange between eastern and western parts, creating conditions that are unpredictable (Zavialov et al. 2009b). All remnants of the former Large Aral have now developed stratified water bodies with anoxic conditions in the deep layers, including the presence of H2S. Methane also has been detected and its release to the atmosphere surpasses levels of most other lakes. These anoxic conditions affect all hydrochemical parameters (Zavialov et al. 2009a; Kurbaniyazov et al. 2018) and obviously also restricts the presence of brine shrimp to the upper layers (Arashkevich et al 2009). The former Tshche-Bas Bay, now an isolated water body in the north-west, represents an intermediate hydrological condition between the Large and the Small Aral Sea (Izhitskaya et al. 2019). In the now semi-isolated Chernyshev Bay the very high transparency of the stratified water has resulted in the development of the world’s largest heliothermal lake,i.e., a water body that acts as a trap of solar energy. Thus, Chernyshev Bay has become a model for tracing biochemical and ecological response to abrupt environmental changes (Izhitskiy et al. 2021).
Irrigation and its side effects
The prime cause for the regression of the Aral Sea was the increased diversion of waters used for irrigation, principally for cotton fields. From the mid 20th century production of cotton along the rivers increased with Turkmenistan and Uzbekistan being the 11th and 8th largest producers on a worldwide basis. Crop area in the Aral Sea basin has actually decreased since 2000 (Huang et al. 2022). But unfortunately much of the water diverted for irrigating the existing cultivated land is lost before being used. This is due both to evaporation from the irrigation canals, from poorly maintained systems in general, and by inadequate management of irrigation techniques. Integrated water management aimed at reducing evapotranspiration and unproductive water losses are crucial to future restoration projects in the area (Huang et al. 2022).
The hotter summers caused by global warming and the disappearance of the Aral Sea entails less precipitation, leading to drier soils and an increasing demand for irrigation of agricultural fields. Furthermore, less water is coming to the two rivers from their sources due to shrinking snow cover and glacier size in the Tien Shan and Pamir mountains. The International Fund for Saving the Aral Sea (IFAS 2022) reports that by 2050 the volume of river runoff in the Amu Darya and Syr Darya rivers will probably be reduced by 10–15% and 2–5%, respectively. Altogether, this means a future with less flow in the rivers, while the demand for water will increase. Predictions are that 5% more water will be needed in 2030, 7–10% in 2050 and 12–16% in 2080. These predictions raise the fear that even the inflow to the reconstituted Small Aral Sea could eventually suffer.
According to the Agency of IFAS (2022) a full reconstitution of the Aral Sea would require an annual supply of 65 km3 of water. Since the demands for agriculture will necessarily continue or are likely to increase, this is clearly impossible. The best hope is for a better use of the available water such as reducing loss from seepage or evaporation in the supply canals and better administration of dosage at the individual plants. In addition, modern agricultural technology using more drought resistant crops is also an option.
Irrigation has already negatively affected both aquatic and terrestrial systems. Fertilizers, pesticides and herbicides from agriculture are washed into the Aral and are being increasingly deposited into the soil which adds to the accumulation of salt in agriculturally irrigated desert areas. Altogether, one might fear that natural terrestrial vegetation could suffer increasingly in the Aral Sea area.
It is thought provoking that the medieval cultures of arid regions were able to economize more wisely with the limited water available. We have not found direct sources proving this for the Amu and Syr Darya areas. But in the similarly arid Iberian peninsula, the farmers in medieval Al Andalus in southern Spain maintained a stable water economy over almost half a millennium (San José 2005; personal communication Puy 2014). Having long experience with agriculture in an arid climate, they used covered supply canals to minimize water loss. In addition, the water was administered by local communities, which provided the people with a direct connection to the limited water supply. Finally, these people did not farm water demanding monocultures but rather food plants that were better suited to the arid climate. Following the Christian takeover of the entire peninsula around AD 1500, the few but ruling Castilian land owners (hidalgos) completely lost interest in farming and its technique. Thus, the link between wise practice and ruler was lost. The parallel to what happened to agriculture in the Aral Sea area in the 20th century is fairly evident even over a historical distance of 500 years.
In recent years, cotton production has decreased somewhat in these countries, due, in part, to water shortage for irrigation in the Amu Darya and in part because agriculture has been redirected to food products for the increasing population. Much water could be saved if irrigation channels were covered and water loss minimized on the way to the fields and drip irrigation used to water the individual plants. The countries in the affected area are increasingly looking to Israel, where such techniques have now entailed a high level of economizing with water (World-nan.kz 2021a b). Climate change has put water resources center stage (Mischke 2020; Mukherji 2022). Solutions should benefit those who face water insecurity, but in addition all possible factors must be considered. Recent studies in the largest contiguous irrigated area in the world (Northwest India and central Pakistan) shows that seepage from canals used for irrigation have contributed to increasing groundwater volume (MacAllister et al. 2022). For the Aral Sea area the situation is different because the amount of water coming through the rivers will decrease and the receiving basin, the Aral Sea, needs to receive as much water as possible. Therefore, any policy to decrease water diversions from the rivers will have a positive effect.
Presently, advanced sensors placed in the field to measure moisture content and ambient temperature are an emerging technique to automatically and economically control water distribution (e.g., Zafar et al. 2020). Much water can also be saved with modern toilets that do not fully flush for every service. It is estimated that such modern systems could save a very large percentage of the water presently being used for irrigation and thus enable continuing farming while also restoring some of the water flow in the rivers. For the Amu Darya, such an increased outflow is vital to any long-term plans for restoring the Large Aral Sea.
Future conservation prospects of the Aral Sea
For the Small Aral Sea, the conditions are now relatively stable but not without challenges. It is highly desirable to prevent an ongoing and significant loss of fish fry in the outflow through the Kokaral Dam, where they are irretrievably lost onto the salt pans. The Small Aral is now also a uniformly brackish water body compared to the diversity of habitats in terms of salinity, depth and temperature range found in the original Aral Sea system. One way to increase habitat diversity entails plans to divide the Small Aral into two lakes: a northern basin on the place of Bolshoy Sarycheganak Bay with a low salinity and a southern basin with a higher salinity (Plotnikov et al. 2021b). This would be accomplished by constructing a new, northerly dam across the entrance of this bay and redirecting some of the water from the Syr Darya into this northern basin by a new canal (Fig. 2). The overflow would then feed the southern basin, still being held stable by the existing Kokaral Dam over the Berg Strait. This project would result in lower salinity conditions in the northern basin and brackish conditions in the southern one. Since the native Aral Sea fauna contained several species or subspecies with different salinity tolerance, such a project would result in a higher biodiversity than with the presently stable but uniform water body. Another plan is to increase the height of the existing dam, thus retaining a single uniform water body but with the benefit of an increase in area and water volume. Both these plans have clear consequences for the resulting biodiversity, including commercially important fish, and it is important that biological insight go together with socio-economic deliberations to arrive at the best possible solution (Plotnikov et al. 2021b).
Aside from the retention and managing of water levels, the Small Aral may also face dangers from the inflowing waters. The intensive agriculture results in run off that contains both fertilizers and possibly unwanted levels of pesticides. General wash out of compounds, such as rare earth elements naturally present in the arid soil, may also be a problem. The extent to which this poses a problem is presently largely unknown.
For the Large Aral Sea plans for improvement would be more costly and difficult to carry out. They would involve channelling waters from the Amu Darya, and ideally also from the Small Aral outflow, to selected areas to restore at least a limited and stable water body (Micklin 2016). Aside from partially restoring the size of the Aral Sea, such efforts would also have beneficial effects upon the climate in the area (He et al. 2022). Unfortunately, prospects are currently unfavourable for an impartial discussion, not to mention any direct action for improving the Small or Large Aral Sea. The present political situation makes this a very secondary issue to most decision makers. To this discussion, some voices opine that the Aral Sea crisis represents effects of climate variations rather than being caused by human activity. While this is unsubstantiated by scientific data, the opinion stems from recent unwillingness to criticize actions occurring during USSR rule. This problem is exacerbated by some local groups who do not welcome any outside interference. Finally, the entire Aral Sea situation involves several countries, both those directly bordering the original sea (Kazakhstan, Uzbekistan) and those through which the two tributary rivers flow and originate (Turkmenistan, Tajikistan, Kyrgyzstan and the People’s Republic of China). As for the World in general, any long-term plans for the water economy of the entire Aral Sea region can only be solved by scientifically informed cooperation and in close association with the needs and hopes of the local populations (Mukherji 2022).
CONCLUSIONS
The regression of the Aral Sea was primarily caused by the ill advised diversion of water for irrigation with no long term considerations of the ecological effects. The resulting crisis involved a complex set of factors that disastrously affected the entire hydrology, biota and even climate of the region, and severely affected the surrounding human population, in terms of both health and economy (Table 9, Fig. 12). This should have served as a warning (UN News 2010), but unfortunately many saline ecosystems in world, most notably the Great Salt Lake, now face a situation with many of the same problems that affected the Aral Sea, including serious threats to human health (Oren 2018; Kintisch 2022; Derouin 2017). Yet it is encouraging that the partial recovery of the Small Aral Sea, by rather modest economical expenditure, almost immediately resulted in a rebounding fishery and improved economy. Although the topology of the Aral Sea was an important factor enabling the re-establishment of a healthy Small Aral, it is nevertheless clear that significant environmental improvement can sometimes be obtained by rather simple means, and have almost immediate effects. At a recent event organized and held by a civil society organization “Turan lowland –Aral Sea,” further improvements of the Aral Sea area, such as those outlined above, were discussed (Ecomarathon 2022). At present, there is no final decision on specific actions, but we hope that plans can be agreed upon across national boundaries for further improvements of the Aral Sea system and its two tributary rivers to the benefit of these ecosystems, including the human population.
Table 9.
Major changes to the Aral Sea fauna during the modern regression. Adapted and extended from Plotnikov et al. (2021b)
Fig. 12.
Causes and effects in the Aral Sea crisis. Hatched outlines show the extension of the sea after the regression had split it into two main bodies in the south and the Small Aral Sea in the north. (1) Diversion of water for irrigation resulted in (2) disastrously reduced inflow to the sea from ca.1960 onwards (3) Almost simultaneously planned species introductions seriously disturbed the native fauna (4) The reduced water inflow caused area regression, increased salinity and therefore serious reduction in biodiversity (5) Almost all fish disappeared with serious economic consequences for the human population (6) Sediment form the dried out sea bottom was blown into the air, causing very serious health problems for the human population (7) At the height of the area regression in 1990, the northern Small Aral Sea was connected to the vanishing Large Aral only through eastern Berg Strait, since the western Auzy-Kokaral Strait had dried out; a dam constructed in 2005 across the Berg Strait now retains inflowing water from the Syr Darya (8) Water level in the Small Aral Sea rapidly increased and salinity decreased; the fauna was reconstituted from refugial populations and fisheries recovered, where it again became an important commercial asset. (9) The larger part of the original Aral Sea continues to degrade into hypersaline water bodies and dried out salt flats with little or no metazoan life (10) The surrounding arid steppe now suffers from more extreme weather oscillations, since Aral waters no longer act as a buffer to temperature (11) In the future, climate caused, decreased precipitation at the sources of the two rivers will add to the shortage of water.
Acknowledgments
This work was supported by the theme of the State assignment for 2022–2024 “Systematization and study of the dynamics of biological diversity and the functioning of ecosystems of continental water bodies under the conditions of anthropogenic impact and climate change” 122031100274-7. We are indebted to Prof. Brian Tsukimura for checking the English language. JTH is grateful to Prof. Carsten Rahbek for many fruitful discussions on biodiversity and conservation issues, without which he would never have ventured into this study.
Footnotes
Authors’ contributions: All authors contributed equally to all aspects of this work.
Competing interests: We declare NO competing interests.
Availability of data and materials: The work is a “review”, so data availability is not an issue.
Consent for publication: YES agreed by all authors.
Ethics approval consent to participate: YES agreed by all authors.
References
- Agency of IFAS. 2022. Crisis of the Aral Sea. Agency of IFAS for implementation of the Aral Sea basin. Available at IFAS. Available at: https://aral.uz/en/crisis/. Accessed 7 Apr. 2022.
- Aladin NV. 1991. Thanatocenoses of separating bays and gulfs of the Aral Sea. Proc Zool Inst Acad of Sci USSR 237:60–63. (in Russian)
- Aladin NV, Kotov SV. 1989. The Aral Sea ecosystem original state and its changes under anthropogenic influences. Proc Zool Inst Acad Sci USSR 199:4–25. (in Russian)
- Aladin NV, Gontar VI, Zhakova LV, Plotnikov IS, Smurov AO, Rzymski P, Klimaszyk P. 2019. The zoocenosis of the Aral Sea: six decades of fast-paced change. Enviro Sci Pollut R 26:2228–2237. doi:10.1007/s11356-018-3807-z. . [DOI] [PMC free article] [PubMed]
- Aladin NV, Plotnikov IS. 1995. Changes in the Aral Sea level: paleolimnological and archaeological evidences. Trudy Zoologicheskogo Instituta 262(1):17–46. (in Russian)
- Aladin NV, Plotnikov IS. 2008. Modern fauna of residual water bodies formed on the place of the former Aral Sea. Trudy Zoologicheskogo Instituta 312(1-2):145–154. doi:10.31610/trudyzin/2008.312.1-2.145. (in Russian)
- Aladin NV, Plotnikov IS, Smurov AO. 2022. Molluscs of the Aral Sea. Biol Bull 49(9):1366–1386. doi:10.1134/S1062359022090023.
- Aladin NV, Potts WTW. 1992. Changes in the Aral Sea ecosystem during the period 1960–1990. Hydrobiologia 237:67–79. doi:10.1007/BF00016032.
- Aladin NV, Plotnikov IS, Smurov AO, Makrushin AV. 2021. Crustaceans of the Aral Sea. Biol Bull 48(7):967–983. doi:10.1134/S1062359021070037.
- Alenitsyn VD. 1875. Otchet o rezul’tatah issledovanii na Aral’skom more [Report on the results of the studies on the Aral Sea]. Trudy Sankt-Peterburgskogo obshchestva estestvoispytatelei [Proceedings of the St. Petersburg Society of Naturalists] 6:72–77. (in Russian)
- Andreeva SI. 1989. Zoobenthos of the Aral Sea in the initial period of its salinization. Proc Zool Inst Acad Sci USSR 199:53–82. (in Russian)
- Andrulionis NY, Zavialov PO, Izhitskiy AS. 2021. Current evolution of the salt composition of waters in the western basin of the South Aral Sea. Oceanology 61(6):899–908. doi:10.1134/S0001437021060035.
- Andrulionis NY, Zavialov PO, Izhitskiy AS. 2022. Modern evolution of the salt composition of the residual basins of the Aral Sea. Oceanology 62(1):30–45. doi:10.1134/S0001437022010027.
- Anchita ZA, Khaibullina Z, Kabiyev Y, Persson KM, Tussupova K. 2021. Health impact of drying Aral Sea: One health and socio-economical approach. Water 13:3196. doi:10.3390/w13223196.
- Arashkevich EG, Sapozhnikov PV, Soloviov KA, Kudyshkin TV, Zavialov PO. 2009. Artemia parthenogenetica (Branchiopoda: Anostraca) from the Large Aral Sea: Abundance, distribution, population structure and cyst production. J Mar Syst 76(3):359–366. doi:10.1016/j.jmarsys.2008.03.015.
- Ataniyazova A. 2003. Health and ecological consequences of the Aral Sea crisis. In: The 3rd world water forum regional cooperation in shared water resources in Central Asia Kyoto, 18 Mar. 2003. Panel III: Environmental Issues in the Aral Sea Basin. Available at https://www.caee.utexas.edu/prof/mckinney/ce385d/Papers/Atanizaova\_WWF3.pdf. Accessed 15 Apr. 2022.
- Bakirov NJ, Khamzaev AK, Novitskiy ZB. 2020. Forest plantations on the drained bottom of the Aral Sea. Lesnoy Zhurnal-forestry Journal 2:51–59. doi:10.37482/0536-1036-2020-2-51-59.
- Behning AL. 1934. Hydrological and hydrobiological materials for fishery map of the Aral Sea. Reports of the Aral-Sea division of the Institute of Marine Fisheries 3:183–205. (in Russian)
- Behning AL. 1935. Materials for fishery map of the Aral Sea. Reports of the Aral-Sea division of the Institute of Marine Fisheries 4:139–195. (in Russian)
- Berg LS. 1908. The Aral Sea. Attempt at a physical-geographical monograph. Stasyulevich Publisher, St. Petersburg. (in Russian)
- Bervald EA. 1950. Biology of the Aral Sea commercial fishes reproduction. In: Materialy po ihtiofaune i rezhimu vod basseina Aralskogo moray. MOIP, Moscow, pp. 83–111. (in Russian)
- Bervald EA. 1964. Ways of organizing rational fish economy on inland water bodies. Rostovskogo Gos Universiteta, Rostov-on-Don, pp. 148. (in Russian)
- Boomer I, Wünnemann B, Mackayc AW, Austinc P, Sorreld P, Reinhardt C, Keyser D, Guichard F, Fontugne M. 2009. Advances in understanding the late Holocene history of the Aral Sea region. Quatern Int 194:79–90. doi:10.1016/J.QUAINT.2008.03.007.
- Borshchov I. 1865. Materials for the botanical geography of the Aral-Caspian region. Zapiski Imperatorskoi Akademii Nauk, Supplement 7(1):1–190. (in Russian)
- Borshchov I. 1877. Algae of the Aral Sea. Supplement to the note by V Alenitsyn “The Aral Sea”. Trudy Aralo-Kaspiiskoy Ekspeditsii. St. Petersburg, p. 38. (in Russian)
- Burr G, Kuzmin YV, Krivonogov S, Gusskov SA, Cruz RJ. 2019. A history of the modern Aral Sea (Central Asia) since the Late Pleistocene. Quaternary Sci Rev 206(1–2):141–149. doi:10.1016/j.quascirev.2019.01.006.
- CBD. 2018. The sixth national report on biological diversity in the republic of Kazakhstan. Prepared by the Secretariat of the Convention of Biological Diversity (CBD), Astana 2018. Available at CBD: https://www.cbd.int/doc/nr/nr-06/kz-nr-06-en. pdf. Accessed 15 Apr. 2022.
- Chen Z, Gao X, Lei JQ. 2022. Dust emission and transport in the Aral Sea region. Geoderma 428:116177. doi:10.1016/j.geoderma.2022.116177.
- Davarpanah S, Erfanian M, Javan K. 2021. Assessment of climate change impacts on drought and wet spells in Lake Urmia basin. Pure App Geophys 178(2):545–563. doi:10.1007/s00024-021-02656-8.
- Deliry SI, Avdan ZY, Do NT, Avdan U. 2020. Assessment of human-induced environmental disaster in the Aral Sea using Landsat satellite images. Environ Earth Sci 79(20):471. doi:10.1007/s12665-020-09220-y.
- Dengina RS. 1954. Data on hydrology and zoobenthos of Muynak Bay of the Aral Sea. Trudy laboratorii ozerovedeniya 3:47–67. (in Russian)
- Dengina RS. 1959. Benthos of Karabaili Archipelago of the Aral Sea. Trudy Laboratorii Ozerovedeniya 8:234–255. (in Russian)
- Derouin S. 2017. Utah’s Great Salt Lake has lost half its water, thanks to thirsty humans. New study suggests consumption— not climate change—is to blame for falling water levels. Science News. doi:10.1126/science.aar3941.
- Dobrokhotova KV. 1971. Some data on productivity of hydromacro-phytes of the Aral Sea. Botanicheskiy Zhurnal 56(1):1759–1771. (in Russian)
- Ecomarathon. 2022. Available at: https://zool.kz/novosti/pridet-li-v-aral-voda-konferencziya-proshedshaya-14-15-oe-maya-v-g-aralsk/. Accessed 13 June 2022.
- Ermakhanov ZK, Plotnikov IS, Aladin NV, Micklin P. 2012. Changes in the Aral Sea ichthyocenosis and fishery in the period of ecological crisis. Lakes & Reserv 17(1):3–9. doi:10.1111/j.1440-1770.2012.00492.x.
- Ermakhanov ZK, Aladin NV, Plotnikov IS. 2013. Evaluation of biological status of main commercial fish species populations in the small Aral Sea. Trudy Zoologicheskogo Instituta 317(Suppl 3):105–112. (in Russian)
- Filippov AA. 1995. Macrozoobenthos of inshore zone of the Aral Sea North in modern polyhaline conditions: quantity, biomass and spatial distribution. Trudy Zoologicheskogo Instituta 262:103–166. (in Russian)
- Fleck J, Udall B. 2021. Managing Colorado River risk. Science 372(6545):885–885. doi:10.1126/science.abj5498. . [DOI] [PubMed]
- Frye RN. 2011. Al-Narshakhi’s The History of Bukhara. Markus Wiener Publishers, Princeton. [translation of: History of Bukhara [Tarikh-i Bukhara] by Abu Bakr Muhammad ibn Jafar Narshakhi. (in Arabic)
- Givati A, Thirel G, Rosenfeld D, Paz D. 2019. Climate change impacts on streamflow at the upper Jordan River based on an ensemble of regional climate models. Journal of Hydrology-Regional Studies 21:92–109. doi:10.1016/J.EJRH.2018.12.004.
- Goaravetisyan. 2021. Berg, Lev Semenovich. Interesting facts. Introduction from: Muromov I. 100 great adventures]. Available at: Goara Vetisyan: https://goaravetisyan.ru/en/berg-lev-semenovich-lev-semenovich-berg-berg-lev-semenovich-interesnye/. Accessed 25 June 2022.
- Gollerbach MM. 1950. Systematic list of charophytes found within the USSR up to 1935 inclusive. Trudy Botanicheskogo Instituta AN SSSR 2(5):20–94. (in Russian)
- Gosteeva MN. 1956. Peculiarities of Aral roach development. Voprosy ichtiologii 6:78–81. (in Russian)
- Gosteeva MN. 1957. Ecologo-morphological characteristics of Aral bream Abramis brama (Berg) development. Trudy Instituta Morfologii Zhivotnykh 20:78–91. (in Russian)
- Gosteeva MN 1959. Spawning and Development of Aral carp on marine spawn area of the Aral Sea. In: Sbornik Rabot po Ihtiologii I Gidrobiologii. Institut Zoologii Akad. Nauk Kazakh. SSR, Alma-Ata, pp. 34–44. (in Russian)
- He HL, Hamdi R, Luo GP, Cai P, Yuan XL, Zhang M, Termonia P, De Maeyer P, Kurban A. 2022. The summer cooling effect under the projected restoration of Aral Sea in Central Asia. Climatic Change 174(1–2):13. doi:10.1007/s10584-022-03434-8.
- Hobbins M, Barsugli J. 2020. Threatening the vigor of the Colorado River. Science 367(6483):1192–1193. doi:10.1126/science. abb3624. . [DOI] [PubMed]
- Huang SY, Chen X, Chang C, Liu T, Huang Y, Zan CJ, Ma XT, De Maeyer P, de Voorde TV. 2022. Impacts of climate change and evapotranspiration on shrinkage of Aral Sea. Science of the Total Environment 845:157203. doi:10.1016/j.scitotenv.2022.157203. . [DOI] [PubMed]
- Husainova NZ. 1960. Narrow bays of the eastern coast of the Aral Sea and their life. Vestn Akad Nauk Kaz SSR 6:34–42. (in Russian)
- Huys R, Herman PMJ, Heip CHR, Soetaert K. 1992. The meiobenthos of the North-Sea -density, biomass trends and distribution of copepod communities. ICES Journal of Marine Science 49(1):23–44. doi:10.1093/icesjms/49.1.23.
- Issayeva AU, Yeshibayev AA, Leska BM, Messyasz B, Abubakirova AA, Tleukeyeva AY. 2021. Comparative assessment of geomorphological and landscape features around the Small Aral Sea. J Ecol Engine 22(10):73–84. doi:10.12911/22998993/142187.
- Izhitskaya ES, Egorov AV, Zavialov PO, Yakushev EV, Izhitskiy AS. 2019. Dissolved methane in the residual basins of the Aral Sea. Environ Res Lett 14(6):065005. doi:10.1088/1748-9326/ab0391.
- Izhitskiy AS, Kirillin GB, Goncharenko IV, Kurbaniyazov AK, Zavialov PO. 2021. The world’s largest heliothermal lake newly formed in the Aral Sea basin. Environ Res Lett 16(11):115009. doi:10.1088/1748-9326/ac2d66.
- Izhitskiy AS, Zavialov PO, Zapozhnikov PV, Kirillin GB, Grossart HP, Kalinina OY, Zalota AK, Goncharenko IV, Kurbaniyazov AK. 2016. Present state of the Aral Sea: diverging physical and biological characteristics of the residual basins. Sci Rep 6:23906. doi:10.1038/srep23906. . [DOI] [PMC free article] [PubMed]
- Katz D. 2022. Basin management under conditions of scarcity: The transformation of the Jordan River basin from regional water supplier to regional water importer. Water 14(10):1605. doi:10.3390/w14101605.
- Karpevich AF. 1960. Biological Basing of aquatic organisms acclimatization in the Aral Sea. Trudy VNIRO 43:76–115. (in Russian)
- Karpevich AF. 1975. Theory and practice of aquatic organisms acclimatization. Pischevaya pronyshlennost, Moscow, p. 432. (in Russian)
- Keith DA, Rodriguez JP, Rodriguez-Clark KM, Nicholson E, Aapala K, Alonso A, Asmussen M, Bachman S, Basset A, Barrow EG, Benson JS, Bishop MJ, Bonifacio R, Brooks TM, Burgman MA, Comer P, Comin FA, Essl F, Faber-Langendoen D, Fairweather PG, Holdaway RJ, Jennings M, Kingsford RT, Lester RE, Mac Nally R, McCarthy MA, Moat J, Oliveira-Miranda MA, Pisanu P, Poulin B, Regan TJ, Riecken U, Spalding MD, Zambrano-Martinez S. 2013. Scientific foundations for an IUCN Red List of Ecosystems, supplementary material. PLoS ONE 8(5):1–25. doi:10.1371/journal.pone.0062111. . [DOI] [PMC free article] [PubMed]
- Kim J, Song C, Lee S, Jo HW, Park E, Yu HN, Cha S, An J, Son Y, Khamzina A, Lee WK. 2020. Identifying potential vegetation establishment areas on the dried Aral Sea floor using satellite images. Land Degrad Dev 31(18):2749–2762. doi:10.1002/ldr.3642.
- Kintisch E. 2022. Record salinity and low water imperil Great Salt Lake. Science 377(6612):1248–1249. doi:10.1126/science. ade8712. . [DOI] [PubMed]
- Khan VM, Vilfand RM, Zavialov PO. 2004. Long-term variability of air temperature in the Aral Sea region. 35th International Liege Colloquium on Ocean Dynamics. J Mar Systems 47(1–4):25–33. doi:10.1016/j.jmarsys.2003.12.006.
- Konovalov MP. 1950. Experiments to study the effect of salinity on the development of roe of vobla, bream and carp. Materials on the ichthyofauna and the water regime of the Aral Sea basin. MOIP, Moscow, pp. 70–82. (in Russian)
- Kortunova TA. 1975. On the changes in the Aral Sea zooplankton in 1959–1968. Zool Zhurn 54:567–669. (in Russian)
- Krijgsman W, Tesakov A, Yanina T, Lazarev S, Danukalova G, Van Baak CGC, Agustím J, Alçiçek MC, Aliyeva E, Bistai D, Bruch A, Büyükmeriç Y, Bukhsianidze M, Flecker E, Frolov P, Hoyle TM, Jorissen EL, Kirscher U, Koriche SA, Kroonenberg SB, Lordkipanidze D, Oms O, Rausch L, Singarayer J, Stoica M, Van de Velder S, Titov VV, Wesseling FP. 2019. Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth Science Reviews 188:1–40. doi:10.1016/j.earscirev.2018.10.013.
- Krivonogov S. 2014. Changes of the Aral Sea level. In: Micklin PN, Aladin NV, Plotnikov I (eds) The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer, Heidelberg, pp. 77–111.
- Krivonogov SK, Burr GS, Kuzmin YV, Gusskov SA, Kurmanbaev RK, Kenshinbay TI, Voyakin DA. 2014. The fluctuating Aral Sea: a multidisciplinary-based history of the last two thousand years. Gondwana Res 26:284–300. doi:10.1016/j.gr.2014.02.004.
- Krivonogov SK, Kuzmin YV, Burr GS, Gusskov SA, Khazin LB, Zhakov EY, Nurgizarinov AN, Kurmanbaev RK, Kenshinbay TI. 2010. Changes of the Aral Sea (Central Asia) in the Holocene: major trends. Radiocarbon 52(2)(special issue 1):555–568. doi:10.1017/S0033822200045598.
- Kumar YA, Sheryzasdanova KG. 2021. Ecotourism study in Kazakhstan: the past, present and the future. Eurasian Journal of Ecology 2(67):4–20. doi:10.26577/EJE.2021.v67.i2.01.
- Kurbaniyazov AK, Makkaveev PN, Makkaveev PN, Zavialov PO, Yusupov B. 2018. Investigation of chemical composition of the Aral Sea water in autumn seasons of 2012 and 2013. News Nat Acad Sci Rep Kazakhstan -Series Geol Tech Sci 3:75–83.
- Lioubimtseva E. 2014. Chapter 7. Impact of climate change on the Aral Sea and its basin. In: Micklin P, Aladin N, Plotnikov I (eds) The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer Earth System Sciences, Springer, Heidelberg, pp. 405–427.
- Liu XZ, Chen H. 2021. Changes of vegetation and its forces driving in the Aral Sea Basin of Central Asia. E3S Web of Conf 269:01013. doi:10.1051/e3sconf/202126901013.
- López-López E. 2021. Editorial: freshwater ecosystems in arid and semiarid zones facing multiple stressors: human disturbances, climate change, and dryland river conservation. Front Environ Sci 9:814225. doi:10.3389/fenvs.2021.814225.
- Loodin N. 2020. Aral Sea: an environmental disaster in twentieth century in Central Asia. Modeling Earth Systems and Environment 6(4):2495–2503. doi:10.1007/s40808-020-00837-3.
- Lukonina NK. 1960. Zooplankton of the Aral Sea. Trudy VNIRO 43(1):177–197. (in Russian)
- MacAllister DJ, Krishan G, Basharat M, Cuba D, MacDonald AM. 2022. A century of groundwater accumulation in Pakistan and northwest India. Nat Geosci 15:390–396. doi:10.1038/s41561-022-00926-1.
- Markovic SB, Ruman A, Gavrilov MB, Stevens T, Zorn M, Komac B, Perko D. 2014. Modeling of the Aral and Caspian seas drying out influence to climate and environmental changes. Acta Geographica Slovenica 54(1):143–161. doi:10.3986/AGS54304.
- Micklin P. 2007. The Aral Sea Disaster. Ann Rev Earth Planetary Sci 35:47–72. doi:10.1146/ANNUREV.EARTH.35.031306.140120.
- Micklin P. 2016. The future Aral Sea: hope and despair. Environ Earth Sci 75:1–15. doi:10.1007/s12665-016-5614-5.
- Micklin P, Aladin N, Plotnikov I (eds). 2014. The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer Earth System Sciences, Springer, Heidelberg. doi:10.1007/978-3-642-02356-9.
- Micklin P, Aladin NV, Chida T, Boroffka N, Plotnikov IS, Krivonogov S, White K. 2020. The Aral Sea: A Story of Devastation and Partial Recovery of a Large Lake. In: Mischke S (ed) Large Asian Lakes in a changing world. Natural state and human impact. Springer, Heidelberg, pp. 109–141. doi:10.1007/978-3-030-42254-7.
- Mischke S (ed). 2020. Large Asian Lakes in a Changing World. Natural State and Human Impact. Springer, Heidelberg, pp. 109–141. doi:10.1007/978-3-030-42254-7.
- Mokievsky VO. 2009. Quantitative distribution of the meiobenthos in the Large Aral Sea in 2003 and 2004. J Marine Systems 76(3):336–342. doi:10.1016/j.jmarsys.2008.03.014.
- Mokievsky VO, Miljutina MA. 2011. Nematodes in meiofauna of the Large Aral Sea during the desiccation phase: taxonomic composition and redescription of common species. Russian J Nematology 19:31–43.
- Mossin J. 1988. Ferrous iron oxidizing bacteria as a food item for Danish Bombina bombina tadpoles at Knudshoved Odde near Vordingborg. Memoranda Soc Fauna & Flora Fennica 64(3):116–118.
- Mukherji A. 2022. Climate change: put water at the heart of solutions. Nature 605:195. doi:10.1038/d41586-022-01273-2. . [DOI] [PubMed]
- Nature Editorial. 2023. The water crisis is worsening. Researchers must tackle it together. Nature 613(7945):611–612. doi:10.1038/d41586-023-00182-2. . [DOI] [PubMed]
- Nikolsky GV. 1940. Fishes of the Aral Sea. MOIP, Moscow, p. 216. (in Russian)
- Nour AM, Vallet-Coulomb C, Bouchez C, Ginot P, Doumnang JC, Sylvestre F, Deschamps P. 2020. Geochemistry of the Lake Chad tributaries under strongly varying hydro-climatic conditions. Aquat Geochem 26(1):3–29. doi:10.1007/s10498-019-09363-w.
- Olafsson E, Carlstrom S, Ndaro SGM. 2000. Meiobenthos of hypersaline tropical mangrove sediment in relation to spring tide inundation. Hydrobiologia 426:57–64. doi:10.1023/A:1003992211656.
- Oren A. 2018. Salt lakes, climate change, and human impact: a microbiologist’s perspective. In: Air and Water -Components of the Environment Conference Proceedings. World Meteorological Day. World Water Day, March, 15-17, 2018, Sovata Romania. Available at http://aerapa.conference.ubbcluj.ro/2018/20\_OREN. htm. Accessed 22 Feb. 2023.
- Orlova MI, Rusakova OM. 1995. Structural and functional characteristics of phytoplanktonic community in the district of Tastubec coge in September 1993. Trudy Zoologicheskogo Instituta 262(1):208–230. (in Russian)
- Ostenfeld CH. 1908. The phytoplankton of the Aral Sea and its affluents with an enumeration of the algae observed. Wissenschaftliche Ergebnisse der Aralsee-Expedition. St. Petersburg 8:123–225, 3 pls, 3 tabs, 3 textfig.
- Pankratova VY. 1935. Materials on the feeding of the Aral Sea fishes. Reports of the Aral-Sea division of the Institute of marine fisheries 43:199–220. (in Russian)
- Pavlovskaya LP. 2022. Fishery in the lower Amu Darya under the impact of irrigated agriculture. Available at FAO. https://www.fao.org/3/v9529e/v9529E04.htm. Accessed 22 Feb. 2022.
- Pavlovskaya LP, Zholdasova IM. 1991. Man-induced changes in fish populations of the Amu-Darya. Vopr Ikhtiol 31(4):585–595. (in Russian)
- Pennisi E. 2015. Just one poorly protected stopover can doom a migrating bird. Conservation efforts fall short for birds that travel long distances. Science Shots Plants & Animals. Available at SCIENCE. https://www.science.org/content/article/just-one-poorly-protected-stopover-can-doom-migrating-bird. Accessed 18 May 2022.
- Pham-Duc B, Sylvestre F, Papa F, Frappart F, Bouchez C, Crétaux J-F. 2020. The Lake Chad hydrology under current climate change. Sci Rep 10:5498. doi:10.1038/s41598-020-62417-w. . [DOI] [PMC free article] [PubMed]
- Plotnikov IS. 2013. Changes in the species composition of the Aral Sea free-living invertebrates (Metazoa) fauna. Trudy Zoologicheskogo Instituta 317(Suppl 3):41–54. (in Russian)
- Plotnikov I, Smurov A, Aladin N. 2021a. Large saline lakes of Central Asia. J Arid Land Studies 31(2):29–44. doi:10.14976/jals.31.2_29.
- Plotnikov IS, Aladin AV, Mossin J, Høeg JT. 2021b. Crustacean fauna of the Aral Sea and its relation to ichthyofauna during the modern regression crisis and efforts at restoration. Zool Stud 60:25. doi:10.6620/ZS.2021.60-25. . [DOI] [PMC free article] [PubMed]
- Plotnikov IS, Ermakhanov ZK, Aladin NV, Micklin P. 2016. Modern state of the Small (Northern) Aral Sea fauna. Lakes Reserv 21(4):315–328. doi:10.1111/lre.12149.
- Puy A. 2014. Land selection for irrigation in Al-Andalus, Spain (8th century AD). J Field Archaeol 39(1):84. doi:10.1179/009346901 3Z.00000000072.
- Radmanesh F, Esmaeili-Gisavandani H, Lotfirad M. 2022. Climate change impacts on the shrinkage of Lake Urmia. J Water Clim Change 13(6):2255–2277. doi:10.2166/wcc.2022.300.
- Reneerkens J, Piermsa T, Spaans B. 2005. The Wadden Sea as a crossroads for bird migration routes. A literature survey on the chances and threats to waterbirds in an international context. NIOZ-Rapport 4:1–125.
- Rusakova OM. 1995. Concise characteristic of the Aral Sea phytoplankton qualitative composition in spring and autumn 1992. Trudy Zoologicheskogo Instituta 262(1):195–207. (in Russian)
- Saidmamatov O, Matyakubov U, Rudenko I, Filimonau V, Day J, Luthe T. 2020. Employing ecotourism opportunities for sustainability in the Aral Sea region: Prospects and challenges. Sustainability 12(21):9249. doi:10.3390/su12219249.
- San José CT. 2005. A social analysis of irrigation in Al-Andalus: Nazari Granada (13th–15th centuries). J Medieval History 31(2):163–183. doi:10.1016/j.jmedhist.2005.03.001.
- Sapozhnikov FV, Ivanishcheva PS, Simakova UV. 2009. Modern assemblage changes of benthic algae as a result of hypersalinization of the Aral Sea. J Mar Systems 76(3):343–358. doi:10.1016/j.jmarsys.2008.03.021.
- Semprucci F, Losi V, Moreno M. 2015. A review of Italian research on free-living marine nematodes and the future perspectives on their use as Ecological Indicators (EcoInds). Mediterr Mar Sci 16(2):352–365. doi:10.12681/mms.1072.
- Stokstad E. 2021. A voice for the river: Jack Schmidt tells hard truths about how climate change will shrink the Colorado River, a lifeline for the Southwest. Will officials listen? Science 373(6550):17–21. doi:10.1126/science.373.6550.17. [DOI] [PubMed]
- Svitoch AA. 2010. Paleogeographical History of the Aral Sea. In: Kostianoy AG, Kosarev AN (eds) The Aral Sea Environment. The Handbook of Environmental Chemistry, vol. 7. Springer, Berlin, Heidelberg. doi:10.1007/698_2009_7.
- The Telegraph. 2010. Aral Sea one of the planet’s worst environmental disasters. The Daily Telegraph, London May 2010. Available at: The Telegraph. https://www.telegraph.co.uk/news/earth/earthnews/7554679/Aral-Sea-one-of-the-planets-worst-environmental-disasters.html. Archived from the original on 8 Apr. 2010.
- UN News. 2010. Shrinking Aral Sea underscores need for urgent action on environment –Ban. United Nations News Global Perspective Human Stories. Available at United Nations News. Available at: https://news.un.org/en/story/2010/04/334402\. Accessed 13 June 2022.
- UN News. 2021. Pink flamingos return to Kazakhstan’s capital in time for world migratory bird day. United Nations News Global Perspective Human Stories. Available at United Nations News. https://news.un.org/en/story/2021/10/1102572\. Accessed 18 May 2022.
- Vergeer HTC, den Hartog C. 1991. Occurrence of wasting disease in Zostera noltii. Aquatic Botany 40(2):155–163. doi:10.1016/0304-3770(91)90093-K.
- Wâhler TA, Dietrichs ES. 2017. The vanishing Aral Sea: health consequences of an environmental disaster. Tidsskrift for Den norske legeforening. 3 October 2017. Available at: https://tidsskriftet.no/en/2017/10/global-helse/vanishing-aral-sea-health-consequences-environmental-disaster. Accessed 20 May 2022. . [DOI] [PubMed]
- Wesselingh FP, Neubauer TA, Anistratenko VV, Vinarski MV, Yanina T, ter Poorten JJ, Kijashko P, Albrecht C, Anistratenko OYu, D’Hont A, Frolov P, Gándara AM, Gittenberger A, Gogaladze A, Karpinsky M, Lattuada M, Popa L, Sands AF, van de Velde S, Vandendorpe J, Wilke T. 2019. Mollusc species from the Pontocaspian region –an expert opinion list. ZooKeys 827:31–124. doi:10.3897/zookeys.827.31365. . [DOI] [PMC free article] [PubMed]
- Whish-Wilson P. 2002. The Aral Sea environmental health crisis. J Rural Remote Environ Health 1:29–34.
- Wikipedia. 2022. Public health problems in the Aral Sea region. Available at: Wikipedia. https://en.wikipedia.org/wiki/Public\_ health_problems_in_the_Aral_Sea_region#Table_1:Infant_ Mortality_Rates,_1985_-2008[11. Accessed 18 May 2022.
- White KD. 2014. Chapter 12. Nature and economy in the Aral Sea Basin. In: Micklin P, Aladin N, Plotnikov I (eds) The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer Earth System Sciences, Springer, Heidelberg, pp. 300–335.
- World-nan.kz. 2021a. Representative of the ministry of agriculture of Israel will study the problems of agro-industrial complex of Kazakhstan. Available at: World-nan. https://world-nan.kz/en/blogs/predstavitel-mskh-izrailya-izuchit-problemy-apk-kazakhstana. Accessed 18 May 2022.
- World-nan.kz. 2021b. Prospects for cooperation between kazakhstan and israel in terms of agriculture. Available at: https://world-nan.kz/blogs/belgiya-rasshirit-kontakty-s-kazakhstanskimi-eksporterami-lna. Accessed 18 May 2022.
- Wucherer W, Breckle SW. 2001. Vegetation dynamics on the dry sea floor of the Aral Sea. In: Breckle SW, Veste M, Wucherer W (eds) State-of-the-art of the ecological research on all problems of land use in various deserts. Springer-Verlag, Berlin Heidelberg, pp. 52–68.
- Yablonskaya EA. 1960. The basis of food production in the Aral Sea and its employment. Trudy VNIRO 42(1):150–176. (in Russian)
- Yablonskaya EA. 1964. On the issue of the importance of phytoplankton and phytobenthos in the food chains of the Aral Sea organisms. In: Marine plant stocks and their uses. Nauka, Moscow pp. 71–91. (in Russian)
- Yang LE, Bork H-R, Fang X, Mischke S, Weinelt M, Wiesehöfer J. 2014. Chapter 1. On the paleo-climatic/environmental impacts and socio-cultural system resilience along the historical silk road. In: Yang LE, Bork H-R, Fang X, Mischke S (eds). Socio-environmental dynamics along the historical silk road. Springer-Verlag, Berlin Heidelberg, pp. 3–22. doi:10.1007/978-3-030-00728-7_1.
- Zafar U, Arshad M, Jehanzeb M, Cheema M, Ahmad R. 2020. Sensor based drip irrigation to enhance crop yield and water productivity in semi-arid climatic region of Pakistan. Pak J Agri Sci 57(5):1293–1301.
- Zavialov PO, Kostianoy AG, Sapozhnikov FV, Scheglov MA, Khan VM, Ni AA, Kudyshkin TV, Pinkhasov BI, Ishniyazov D, Petrov MA, Kurbaniyazov AK, Abdoullaev UR. 2003. Modern hydrophysical and hydrobiological state of the western part of the Aral Sea. Okeanologiya 43(2):316–319.
- Zavialov PO, Ni AA, Kudyshkin TV, Ishniyazov DP, Tomashevskaya IG, Mukhamedzhanova D. 2009a. Ongoing changes of ionic composition and dissolved gases in the Aral Sea. Aquat Geochem 15(1–2):263–275. doi:10.1007/s10498-008-9057-9.
- Zavialov PO, Ni AA, Kudyshkin TV, Kurbaniyazov AK, Dikarev SN. 2009b. Five years of field hydrographic research in the Large Aral Sea (2002–2006). J Mar Systems 76(3):263–271. doi:10.1016/J.JMARSYS.2008.03.013.
- Zhakova LV. 1995. Tolypella spicata (Nitellaceae): A new Charophyta species for the Russian flora. Botanicheskii Zhurnal (St. Petersburg) 80(8):109–112. (in Russian)
- Zhakova LV. 2013. Effect of long-term changes of the salinity on the water flora composition and distribution of macrophytes in the Aral. Trudy Zoologicheskogo Instituta 317(Suppl. 3):113–119. (in Russian)
- Zhitina LS. 2011. Phytoplankton of the Large Aral Sea in June 2008. Oceanology 51(6):1004–1011. doi:10.1134/S0001437011060233.
- Zonn IS. 2009. Social, economic, legal and political issues of the Russian Arctic. In: Nihoul JCJ, Kostianoy AG (eds) Influence of Climate Change on the Changing Arctic and Sub-Arctic Conditions. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht, pp. 209–220. doi:10.1007/978-1-4020-9460-6_15.