Breathing Practices for Stress and Anxiety Reduction: Conceptual Framework of Implementation Guidelines Based on a Systematic Review of the Published Literature (original) (raw)
Abstract
Anxiety and stress plague populations worldwide. Voluntary regulated breathing practices offer a tool to address this epidemic. We examined peer-reviewed published literature to understand effective approaches to and implementation of these practices. PubMed and ScienceDirect were searched to identify clinical trials evaluating isolated breathing-based interventions with psychometric stress/anxiety outcomes. Two independent reviewers conducted all screening and data extraction. Of 2904 unique articles, 731 abstracts, and 181 full texts screened, 58 met the inclusion criteria. Fifty-four of the studies’ 72 interventions were effective. Components of effective and ineffective interventions were evaluated to develop a conceptual framework of factors associated with stress/anxiety reduction effectiveness. Effective breath practices avoided fast-only breath paces and sessions <5 min, while including human-guided training, multiple sessions, and long-term practice. Population, other breath paces, session duration ≥5 min, and group versus individual or at-home practices were not associated with effectiveness. Analysis of interventions that did not fit this framework revealed that extensive standing, interruptions, involuntary diaphragmatic obstruction, and inadequate training for highly technical practices may render otherwise promising interventions ineffective. Following this evidence-based framework can help maximize the stress/anxiety reduction benefits of breathing practices. Future research is warranted to further refine this easily accessible intervention for stress/anxiety relief.
Keywords: breathing, breathwork, pranayama, respiration, diaphragmatic, stress, anxiety
1. Introduction
The rising prevalence of anxiety and chronic stress plagues populations worldwide. Prior to the COVID-19 pandemic, anxiety disorders had already attained startlingly high proportions, affecting nearly one-third of Americans at some point during their lifetime [1]. Since then, anxiety disorders have surged by an estimated 25.6% globally [2] and they burden the economy with healthcare costs and reduced productivity [3,4]. Both above and below the clinical threshold, anxiety and chronic stress impact physical, mental, and cognitive health, increasing the risk of cardiometabolic disorders, cancer, mental illness, neurodegenerative disease, and all-cause mortality [5,6,7,8,9,10,11,12,13,14]. Chronic activation of the stress response induces allostatic overload, a cumulative “wear and tear” on the brain and body that erodes resiliency and health [15].
Common stress and anxiety treatments rely on external factors, such as a therapist or medication, and come with modest effect sizes [16] or unfavorable side effects [17]. Effective, accessible, and risk-free solutions are needed.
Voluntary regulated breathing practices may offer such a solution. These practices draw on both modern scientific studies and ancient yogic-pranayama concepts, with applications ranging from clinical anxiety treatment in adults to reducing stress reactivity in college athletes [18,19,20,21,22]. Common voluntary regulated breathing practices include diaphragmatic breathing, paced slow breathing, breathing with biofeedback, and alternate-nostril breathing (ANB). Breathing practices, when used in isolation, have the advantage of being universally accessible, scalable, and cost-free. They are not limited by access to healthcare services nor burdened by side effects and put potential treatment tools in the hands of the individual [23,24,25].
Breathing practices’ effects on the autonomic nervous system and brain may underlie their stress-reducing benefits. Effective breathing interventions support greater parasympathetic tone, which can counterbalance the high sympathetic activity intrinsic to stress and anxiety. Respiratory entrainment of brain rhythms offers an additional avenue through which breathing may influence neural circuit dynamics, cognition, and mood [26,27]. Breathing also uniquely engages in a reciprocal relationship with stress and anxiety, whereby stress and anxiety can both affect [28,29,30,31,32,33] and be affected by [20,21,22,34] altered respiratory patterns. Due to the shared neurophysiological underpinnings of stress and anxiety, from hereon, the term “stress” is used to encompass both conditions.
Although existing literature confirms the widespread benefits of slow breathing practices for stress/anxiety reduction [23,24,25,35,36,37,38], null or negative results are reported in some studies [39,40,41,42,43,44,45,46,47,48,49,50]. Discerning the distinguishing features between effective and ineffective interventions is of interest.
We conducted a systematic review of clinical studies that evaluated the effectiveness of voluntary regulated breathing practices on psychometric measures of anxiety and stress. We identified intervention characteristics associated with effectiveness and used these data to build a conceptual framework of components associated with the stress reduction effectiveness of breathing practices.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were strictly followed throughout. A protocol was not developed or registered.
2.1. Overview
We searched peer-reviewed, published literature for original clinical trials evaluating the effectiveness of voluntary regulated breathing practices on psychometric stress and anxiety measures. Our search covered articles published through March 2023, written in English, and from any geographic location. We operationally defined “voluntary regulated breathing practice” as any voluntary, regulated manipulation or control of the breath. We chose a broad and inclusive definition in an effort to capture a variety of regulated breathing methods, including deep breathing, diaphragmatic breathing, yoga or pranayama breathing, slow breathing, paced or patterned breathing, ANB, and more.
2.2. Search Strategy
Our search strategy incorporated PubMed and ScienceDirect electronic searches (Appendix A.1) and manual reference-mining of previously published reviews and key clinical trials [22,23,24,25,33,35,36,37,38,51,52,53,54,55,56,57,58,59,60,61].
2.3. Selection Criteria
2.3.1. Publication and Study Type
We included peer-reviewed, published randomized controlled trials (RCT) and other non-randomized prospective cohort and clinical studies. Gray literature, case studies, retrospective observational data analyses, editorials, and reviews were excluded.
2.3.2. Intervention
We included studies that considered any voluntary, regulated breathing practice as its own intervention, whether part of the treatment or control. We excluded interventions that used breath practices in combination with other interventions, such as yoga, meditation, tai chi, etc. We included interventions that used metronomes or visual/audio cues for guiding breathing pace and excluded those in which a device provided real-time biofeedback, such as data on expelled carbon dioxide (CO2), respiratory rate, heart rate variability (HRV), etc. We excluded interventions that incorporated means outside of participants’ direct control to manipulate or regulate participants’ oxygen or CO2 intake.
2.3.3. Outcomes
We included studies where primary or secondary outcomes incorporated psychometric stress or anxiety measures, defined as those that quantitatively measure stress or anxiety by use of inventory, questionnaire, observation, or self-report. Studies that evaluated fear outcomes or physiologic measures were included only if psychometric measures of stress/anxiety were also included.
2.3.4. Populations
The review included participants with clinical stress or anxiety (e.g., anxiety disorders, post-traumatic stress disorder) and non-clinical stress (e.g., healthy adults, youth, disease-specific populations). Studies with participants aged ≥1 year (i.e., capable of intentional breath regulation), of both genders and of any education or socioeconomic status were included.
2.4. Article Screening and Data Extraction
Articles were reviewed in the following stages:
- Title-screening;
- Abstract-screening of accepted titles;
- Full-text screening of accepted abstracts;
- Data extraction from accepted articles.
Two reviewers conducted all levels of screening and data extraction. Discrepancies were resolved through discussion or review by a third reviewer.
Data extracted from accepted articles included journal and publication information, participant demographics, inclusion and exclusion criteria, control and intervention descriptions, study duration, outcome measures, results, limitations, and implications (Appendix A.2). Data collected for framework development comprised details about study design, breath practice, intervention duration, and implementation components, such as use of human-guided training, number of sessions, and use of long-term practice. We defined human-guided training as the use of live or pre-recorded audio or video human guidance during at least the first breath session. Multiple sessions were defined as performing the breathing practice more than once, whether separated by minutes or multiple days. Long-term practice was defined as ≥6 sessions over ≥1 week.
Authors were contacted when data were unavailable or unclear.
2.5. Assessment of Study Quality
Studies were assessed by two independent reviewers for methodological and reporting quality. Disagreements between reviewers were resolved through discussion or by a third reviewer. RCTs were assessed using a modified form of the Boutron checklist to evaluate a report of a nonpharmacological trial (CLEAR-NPT; Appendix A.3) [51,62]. Non-RCTs were assessed using the National Heart, Lung, and Blood Institute of the National Institutes of Health (NHLBI-NIH) quality assessment tool for single-arm, pre-post, or non-randomized studies (Appendix A.4) [63]. For both tools, scores <0.6 were considered poor quality, 0.6–0.8 fair, and >0.80 good.
All selected studies were included in data extraction and synthesis, regardless of their methodological quality, as assessed with these tools.
2.6. Synthesis
Results were synthesized by first classifying the studies by study population, type, and use of stress/anxiety as a primary versus secondary outcome. Patterns among effective and ineffective interventions were identified based on study design and population and breath practice type, training, duration, and implementation. We compared the frequencies of these data to identify factors associated with intervention effectiveness. Parameters were categorized in n × 2 contingency tables where rows represented parameters and columns represented effectiveness. Fisher’s exact tests of independence [64,65] were performed for each parameter that could be summarized in a contingency table with fixed margins. Interventions for which effectiveness outcomes differed from the above patterns were labeled outlier interventions, details of which were explored through qualitative analysis to provide further insights into effectiveness patterns. Synthesis results were used to create a conceptual framework of intervention characteristics associated with stress reduction effectiveness. Analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021). Results were presented in narrative, tabular, and figure form.
3. Results
3.1. Study Selection and Characteristics
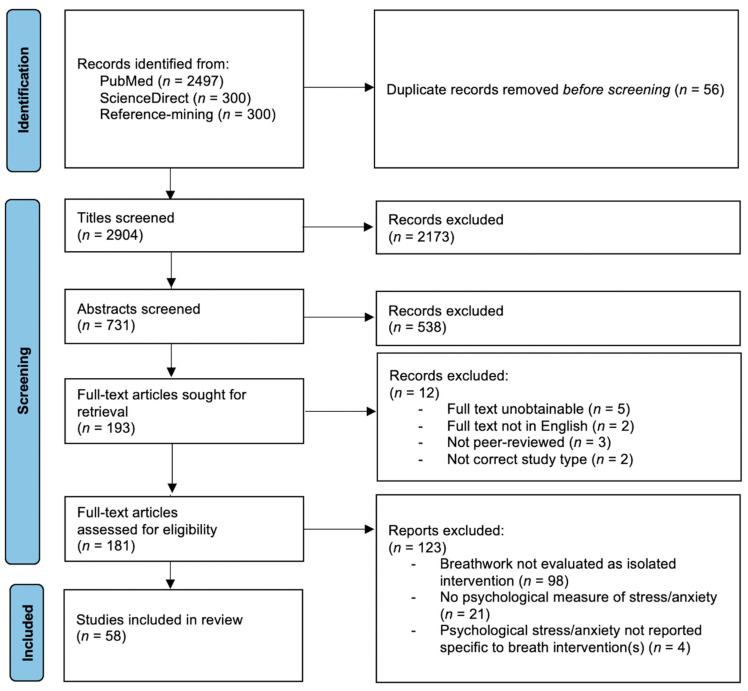
Results of the search and screening process are presented in the PRISMA flow diagram (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study identification, screening, and selection process. n, number.
Table 1 provides details of all included studies by population and by use of stress/anxiety as the primary or secondary outcome. Six broad population categories emerged: youth (4 studies); healthy adults (14 studies); high-anxiety adults (4 studies); clinical populations (29 studies), divided into chronic-care clinical populations (9 studies), such as those with ongoing asthma or hypertension, and acute-care clinical populations (20 studies), such as those undergoing chemotherapy, dental care, or surgery; and individuals placed in simulated-stress situations (7 studies), such as an artificial public speaking task or electric shock anticipation. Forty-one of the 58 studies were RCTs, and 17 were non-randomized prospective clinical studies. Of the 41 RCTs, 23 included psychometric measures of stress or anxiety as primary outcomes, and 18 included these measures as secondary outcomes. Of the seventeen non-RCTs, 8 included psychometric measures of stress or anxiety as primary outcomes, and 9 did as secondary outcomes. Forty-four of the 58 studies were comparative; comparators were usual care or no intervention in 22 studies, other breath-related interventions in 8 studies, and non-breath-related interventions in 14 studies.
Table 1.
Details of included studies’ design and interventions (N = 58) by population and use of stress/anxiety as primary or secondary outcome.
| Author, Year, Setting | Study Design, Population | Mean Age (SD),% Female,N | Intervention and Control,Time Frame |
|---|---|---|---|
| A. Youth | |||
| Youth: Stress/anxiety as primary outcome | |||
| * Sellakumar, 2015 [20] India, secondary school (grades 6–12) | RCTAdolescent school students | 14 y, 48%Ix: 50C: 50 | Ix: 1-time guided group training followed by 45 days of 30 min individual: rhythmic breathing, pranayama, SK, slow breathing, deep breathing, prolonged exhales, brief breath-holdsC: No intervention |
| * Khng, 2017 [66] Singapore, 4 elementary schools | RCT5th-grade students | Ix: 10.7 y, 48%, 61C: 10.7 y, 48%, 61 | For 10 min prior to and during six 1 min breaks in 2nd administration of math test:Ix: Deep abdominal breathing; C: Resting quietly |
| * Bargale, 2021 [67]India, hospital pediatric dentistry dept. | RCTChildren needing dental treatment under anesthesia | 51%Ix: 8.5 y (1.9), 30C: 8.4 y (1.9), 30 | Two sessions, before and after anesthetic procedure, of guided:Ix: Slow inhale, 2 s hold, exhale into pinwheel C: Relaxation breathing, feeling chest and belly move |
| Youth: Stress/anxiety as secondary outcome | |||
| * Hakked, 2017 [68] India, swim academy | RCTCompetitive youth swimmers | 52%Ix: 15.2 y, 14C: 15.1 y, 13 | For 4 wks, regular swim training alongside:Ix: 10 m, 5×/wk in group w/audio-guided yogic breathing practices:* Sectional (abdominal, thoracic, upper lobar) breathing* Yogic bellows breathing (forceful nasal breathing w/o strain and w/abdominal expansion/contraction)* ANB w/breath retention after inhaling C: No intervention |
| B. Healthy Adults | |||
| Healthy Adults: Stress/anxiety as primary outcome | |||
| * Gupta, 2010 [69]India, yoga training camp | Pre-post trialMarried men | 60–70 y (range), 0%, 30 | 7-day group training + 3 mo individual practice of ANB |
| * Bhimani, 2011 [31] India, medical college | Pre-post trialNewly admitted medical students | 17–22 y (range), 54%, 59 | 8 wks of 1 h/day, 5 days/wk instructor-led group pranayama: Kapalabhati, External Kumbhaka (Bahya), Easy Comfortable Pranayama (Sukha Purvaka), Surya Bhedan, Ujjayi, Sitkari, Sitali |
| * Sharma, 2013 [70]India, yoga research center and school | RCTHealthy healthcare students | Ix-1: 18.4 y, 77%, 30Ix-2: 19.2 y, 87%, 30C: 18.9 y, 83%, 30 | For 12 wks, 30 m/day, 3×/week, of guided group:Ix-1: Fast pranayama—repeated cycles of 1 min each kapalabhati, Bhastrika, kukkriya, then 1 min restIx-2: Slow pranayama—repeated cycles of 2 min each of ANB, 6-3-6-3 breath, extended exhales w/sounds, then 1 min rest* Ix-1 and Ix-2 both end w/10 min shavasana C: No intervention |
| * Sundram, 2014 [71]Malaysia, automotive assembly plant | Pre/post-test nonequivalent-groups designMale automotive assembly line workers | 20+ y (range)Ix: 20+ y, 0%, 468C: 20+ y, 0%, 293 | Ix: Five 10 min group sessions of guided deep breathing over 9 mo, +2×/day at-home practice encouragedC: Pamphlet on stress and its ill-effects, w/no stress reduction technique info |
| * Schmalzl, 2018 [72] US, university town | RCTYoga-naive healthy adults | Ix: 25 y, 54%, 22C: 24 y, 61%, 18 | 8-wk, 2×/wk instructor-led group classes + remaining days 15–20 min video-guided group practice:Ix: Yoga + breathing, equal-paced inhales/exhales, movements paced to breathing, 5 breaths in each poseC: Seated breath practice, visualizing breath moving up and down spine, directing breath to specific body parts, short breath holds after inhale/exhale, and ANB |
| * Okado, 2020 [73]US, university setting | RCTUndergraduate students | 19.81 y (2.42)Ix-1: 20.16 y (3.80), 45.5%, 20Ix-2: 19.40 y (1.75), 72.1%, 31Ix-3: 19.68 y (1.74), 68.2%, 30C: 19.99 y (2.37), 79%, 64 | 1 in-person session followed by 2 wks of individual practice:Ix-1: Guided diaphragmatic breathingIx-2: Relaxation techniques, asked to identify relaxation strategies in session Ix-3: Identical to Ix-2, +activity handout w/behavioral strategies to promote relaxationC: No intervention |
| * Magnon, 2021 [74]France, setting unclear | Single-arm studyHealthy older and young adults | 41.2 y (23.6), 80.9%, 47Older: 65.9 y (5.1)Young: 19.6 y (1.6) | Single 5 min session of slow breathing following on-screen moving drop of water; starting w/4 s inhale/exhale, gradually extending exhale to 6 s |
| * Balban, 2023 [75]US, remote setting | RCTAdults w/o severe psychiatric or medical conditions | 27.97 y, 68.5%Ix: 84C: 24 | 28 days of daily 5 min home practice:Ix-1: Cyclic sighing: inhale slowly, inhale once more, slowly exhale all breathIx-2: Box breathing: individualized pace w/equal length inhale, exhale, and breath holdsIx-3: Cyclic hyperventilation w/retention: 3 rounds of 30 deep inhales w/passive exhale, ending w/15 s holdC: Mindfulness meditation: sit/lie down, close eyes, breathe, focus on forehead between eyes |
| Healthy Adults: Stress/anxiety as secondary outcome | |||
| * Busch, 2012 [76] Germany, university setting | Pre-post trial of 2 Ix’s separated by 6 moHealthy undergraduate students | 25 y, 87%, 15 | 6 weekly sessions of 20 min instructor-guided DSB, 7 bpm:Ix-1: DSB alone; Ix-2: DSB w/attentive concentration respiratory feedback task |
| Lin, 2014 [45]Taiwan, university setting | Within-subjects, 4-arm trialHealthy college students | 20.98 y (1.03), 78.7%, 47 | 5 min sitting then paced breathing using on-screen pacer for 2 min per pattern w/1 min rest between Ix-1: 6 bpm, 5-0-5-0; Ix-2: 6 bpm, 4-0-6-0; Ix-3: 5.5 bpm, 4.4-0-6.6-0; Ix-4: 5.5 bpm, 5.5-0-5.5-0 |
| * Hunt, 2018 [21] US, large private university | RCTCollegiate varsity soccer, swimming, track athletes | 20 y, 59%, 76 | 10 min metronome-guided paced breathing at 6 bpm followed by either:Ix: 5 min guided + 5 min unguided DB, aiming for 4 bpmC: 10 min guided PMRAfterwards, all did 5 min paced breathing (6 bpm) followed by cognitive stressor challenge |
| * Naik, 2018 [77]India, medical school | RCTHealthy adult male volunteers | Ix: 24.45 y, 0%, 49C: 24.12 y, 0%, 50 | For 12 wks:Ix: 5×/wk of 30 min guided modified ANB (6-6-6-0) + instructions to practice at home other 2 days/wkC: No Ix |
| * Conlon, 2022 [78]UK, University of Bath | RCTCollegiate and National Team level athletes w/no formal shooting experience | 20.17 y (2.77)Ix-1: 60.9%, 23Ix-2: 60.9%, 23C: 57.1%, 21 | Between 2 shooting task exercises, 5 min guided training + 5 min pacer-guided practice w/nasal inhales, pursed-lip exhales of:Ix-1: 4 s inhale, 6 s exhale (6 bpm) diaphragmatic breathingIx-2: 2.5 s inhale, 2.5 s exhale (12 bpm) paced breathingC: Watched 10 min respiratory anatomy educational video |
| * Schlatter, 2022 [79]France, university medical school | RCT5th-yr anesthesiology and critical care residents participating in simulations for medical training | 29 y (1) Ix-1: 50%, 12Ix-2: 27%, 11C: 46%, 11 | For 5 min before engaging in 15 min simulated medical scenario w/performance evaluation:Ix-1: Relaxing breathing w/4 s inhale, 6 s exhale, following moving cursor on computerIx-2: Same as Ix-1, but w/HRV biofeedback and instructions to increase HRVC: Read lab test results |
| C. High-Anxiety Populations | |||
| High-Anxiety Populations: Stress/anxiety primary outcome | |||
| * Clark, 1990 [80] US, inpatient alcohol rehabilitation facility | RCTMale, alcohol-dependent inpatients scoring high trait anxiety (STAI > 46.62) | 39.3 yIx: 0%, 18C: 0%, 18 | Two 10 min sessions, 2 days apart, w/audio pacing tones in 1st session and no sound in 2nd:Ix: Guided slow breathing (10 bpm) at audio tone pace session 1; at same yet self-guided pace session 2C: Count audio tones session 1, relax session 2 |
| * Chen, 2017 [81]Taiwan, medical center | RCTHigh-anxiety adults from outpatient psych unit | Ix: 24 y (6), 60%, 15C: 25 y (7), 73%, 15 | 12 individual training sessions over 8 wks + 2×/day home practice of:Ix: Guided diaphragmatic breathingC: “Routine respiration” |
| * Serafim, 2018 [82] Brazil, hospital | Pre-post open-label uncontrolled clinical trialOutpatients w/stable BD and complaints of anxiety | 42.8 y, 70%, 14 | Ix: 4 weekly 1:1 guided deep breathing trainings of 7, 10, 15, and 20 m, respectively, followed by at-home practice for 1 wk |
| High-Anxiety Populations: Stress/anxiety as secondary outcome | |||
| * Clark, 1985 [83] UK, setting unclear | AB case seriesOutpatient adults with frequent panic attacks and associated anxiety | 36 y, 72%, 18 | Ix: 2 sessions, w/1 wk between, of 2 min voluntary hyperventilation+discussion of over-breathing and panic attacks, followed by guided slow breathingBefore and after Ix, 11 were exposed to individually determined anxiety-provoking situation |
| D. Clinical Populations (Acute) | |||
| Clinical Populations (Acute): Stress/anxiety as primary outcome | |||
| Biggs, 2003 [40] US, private dental practice | RCTPatients seeking dental treatment | 38 y,58%,272 | Written instruction in waiting room:Ix-1: Unguided deep diaphragmatic breathingIx-2: Focused attention on non-teeth body partC: No intervention |
| * Hayama, 2012 [84]Japan, hospital inpatient | RCTWomen w/recent Dx of gynecological cancer undergoing 1st chemo Tx | Ix: 53.6 y (9.4), 100%, 11C: 61.7 y (9.8), 100%, 12 | Ix: Before 1st chemo session and on days 2, 4, 6 post-chemo, 10 min of 4-step individual guided diaphragmatic breathing of 10 deep inhales and slow exhales per step, done lying down: breaths 0–10 and 30–40 w/arms raised; 11–20 abdominal and 21–30 thoracic breathingC: Usual chemo + nursing care |
| * Valenza, 2014 [85] Spain, hospital | RCTCOPD exacerbation patients | Ix: 76 y, 0%, 23C: 74.4 y, 0%, 23 | Ix: 10-day, 2×/day 30 m, guided breathing of inspiratory muscle relaxation, pursed lip breathing, prolonged and active expirationC: Standard care |
| * Bidgoli, 2016 [86]Iran, hospital | RCTPatients undergoing first coronary angiography and experiencing anxiety | Ix: 55.5 y, 55%, 40C: 62.7 y, 45%, 40 | Ix: Single session before surgery of 5 min guided sukha pranayama—slow, rhythmic breathing w/equal length inhales-exhalesC: Routine care |
| Boaviagem, 2017 [41] Brazil, maternity hospital | RCTNulliparous women in active labor, 37–41 wks gestation | Ix: 21.2 y, 100%, 60C: 20.6 y, 100%, 61 | Ix: Instruction to use slow deep breathing (5 s inhales, 5 s pursed lip exhales) during dilation, active, late labor phases, including post-exhale pause (1–2 s) for active and late active labor onlyC: Standard care |
| * Cicek, 2017 [87] Turkey, hospital delivery room | RCTNulliparous women 38–42 wks pregnant w/o complications in early labor | Ix: 23.3 y, 100%, 35C: 22.4 y, 100%, 35 | Ix: 30 min guided Lamaze breath stages for progressive labor w/real-time guidance during labor of normal nasal, slow deep chest, rapid shallow, rapid abdominalC: Routine hospital checks only |
| * Chandrababu, 2019 [88] India, hospital | Non-randomized 2-arm trialPatients undergoing 1st-time cardiac surgery | 58.7 yIx: 25%, 24C: 29%, 24 | Ix: 15 min guided ANB, taught 2 days prior to surgery and performed on days 3–5 post-surgeryC: Usual pre- and post-operative care |
| Ratcliff, 2019 [47]US, university medical center | RCTWomen scheduled for stereotactic breast biopsy | 55.4 y (11.27)Ix-1: 55.1 y, 100%, 30 Ix-2: 55.1 y, 100%, 30 C: 55.9 y, 100%, 16 | 10 min prior to and throughout biopsy, guided:Ix-1: Mindfulness meditation; Ix-2: Diaphragmatic breathingC: Listened to neutral-content audio |
| * Grinberg, 2020 [89]US, medical center | Non-randomized pre-post studyMale veterans scheduled for prostate biopsy | 66.9 y (6.46) Ix: 66.7 y, 0%, 20C: 65.05 y, 0%, 20 | Ix: 15 min guided diaphragmatic breathing immediately prior to biopsyC: Standard care |
| * Abo El Ata AB, 2021 [90]Egypt, hospital | Pre-post studyBurn patients during dressing changes | 28.8 y (8.9), 37%, 7 | 2 wk daily guided relaxation breathing exercises before and during daily dressing change |
| * Hosseinzadeh-Karimkoshteh, 2021 [91]Iran, medical center | RCTBurn patients | Ix: 25.5 y, 33.3%, 15C: 28.1 y, 40%, 15 | 4 days of:Ix: 30 min guided relaxation breathing before dressing change, 4-4-4-0 pursed-lip exhalesC: Routine care |
| * Zahn, 2021 [92]Switzerland, university hospital | RCTAdults having dermatological surgery w/local anesthesia | Ix: 60.7 y (20.6), 40.8%, 86C: 60.2 y (18.9), 48.8%, 84 | Before undergoing surgery:Ix: Deep breathing instruction, were told to perform before and during procedureC: No Instruction |
| * Moghadam, 2022 [93]Iran, psychiatric hospital | RCTAdults receiving ECT for depression | Ix-1: 35 y (14), 50%, 30Ix-2: 38 y (9), 53.3%, 30C: 36 y (10), 50%, 32 | Before ECT procedure:Ix-1: 10 min guided slow breathing, (5 s deep nasal inhale, 5 s mouth exhale)Ix-2: 3–5 min breathing lavender aromatherapy oilC: Routine care |
| * Aktas, 2023 [39]Turkey, Ankara Hospital | RCTPostoperative bariatric surgery patients | 34.7 yIx-1: 76.7%, 23Ix-2: 66.7%, 20C: 53.3%, 16 | Each h during post-op h’s 1-6:Ix-1: 4-7-8 breathing technique, 10 breaths; Ix-2: Deep Breathing, 4 breathsC: Treatment as usual |
| Clinical Populations (Acute): Stress/anxiety as secondary outcome | |||
| * Dhruva, 2012 [94] US, university medical center | RCT w/single-arm crossoverCancer patients undergoing intravenous chemo | Ix: 52 y, 75%, 8C: 56 y, 100%, 8 | During 2 consecutive chemo cycles:Ix: Usual care + 60 min class 1×/wk + 2×/day of 10–15 min individual home pranayama:* breath observation* ujjayi breathing: slow, deep abdominal rhythmic inhales/exhales, w/extended exhales and partially closed glottis* kapalabhati breathing: gentle inhales, brief pause, forceful exhale* ANBC: Usual care for 1st chemo cycle; usual care + Ix for 2nd cycle |
| * Park, 2013 [95]South Korea, medical center | Pre-post study comparison groupBurn patients | 44.9 yIx: 44.5 y, 43.3%, 30C: 45.3 y, 60%, 30 | Over 3 days: Ix: Guided relaxation breathing 4-0-4-0 before and 4-0-2-0 during dressing changesC: Usual dressing change procedure |
| * Eldin, 2015 [96]Libya, hospital | RCTBurn patients | Ix: 18–50 y, 45%, 20C: 18–50 y, 30%, 20 | Over 3 days:Ix: 15+ min guided relaxation breathing (in-nose, out-mouth)C: Standard care |
| * Iyer, 2020 [97]India, medical center | Pre-post Burn patients | 31.8 y (17.8), 35%, 20 | Over 7 days:Ix: 15 m/day guided deep breathing |
| * Ursavas, 2020 [98]Turkey, hospital | RCTTotal knee replacement patients | Ix: 65 y, 79%, 19C: 69 y, 79%, 19 | Ix: Guided diaphragmatic breathing + instruction to use at 1, 2, 4, 8, 12, and 24h post-op + when experiencing pain/anxietyC: Standard care |
| * Lu, 2022 [99]China, hospital | RCTLung cancer patients undergoing thoracic surgery | 59.3 y (10.2)Ix: 55%, 34C: 61%, 34 | Over 14 days:Ix: 2×/day of 15–20 min guided Active Cycle of Breathing Technique, each part repeated 3–5×: slow inhale, 3 s hold, 6 s pursed-lip exhale; deep active inhale, 3 s hold, passive exhale (all nasal; slow deep nasal inhale, 2–3 forceful “huff” mouth exhales)C: Usual care |
| E. Clinical Populations (Chronic) | |||
| Clinical Populations (Chronic): Stress/anxiety as primary outcome | |||
| * Marshall, 2014 [100] US, setting unclear | Pre-post repeated-measures trialPost-stroke individuals w/brain damage, w and w/o aphasia | 55.6 y, 18%, 11 | 4 wks of 1 h guided practice 1×/wk, followed by 6 wks of 5–40 min independent practice of right UNB: occlude left nostril while inhaling and exhaling through the other, work toward exhaling twice as long as inhale |
| Clinical Populations (Chronic): Stress/anxiety as secondary outcome | |||
| * Han, 1996 [22] Belgium, setting unclear | Pre-post single-arm trialAdults w/HVS | 37 y, 65%, 92 | 17 sessions over 2.5 mo of physiotherapist-guided breathing therapy and retraining:* 1st session: 3 min voluntary hyperventilation at 60 bpm followed by therapy to reattribute cause of daily life complaints to hyperventilation and explain breath retraining purpose* Remaining sessions: 45 min breath retraining w/abdominal breathing and slowed exhales |
| * Thomas, 2009 [101] UK, primary care general practices | RCTAdults w/asthma and impaired health status | Ix: 46 y (median), 58%, 66 C: 46 y (median), 64%, 63 | Ix: 3 physiotherapist-guided sessions w/2–4 wks between each:* One 1 h small group breathing education session* Two 30–45 min individual DB and nasal breathing training sessions* Participants instructed to practice breathing exercises ≥10 m/dayC: Asthma nurse-delivered education |
| Jefferson, 2010 [43]US, setting unclear | RCTAfrican-American females w/hypertension | Ix: 54 y, 100%C: 56 y, 100% | Ix: 20 min therapeutic chair massage_C:_ Education on DB |
| * Sureka, 2014 [102] India, prison hospital | RCTAdult male prisoners w/psychiatric disorder | Ix: 36 y, 0%, 116C: 36 y, 0%, 115 | For 6 wks, 30 m/day:Ix: Guided SKY breathing: 8 min Ujjayi, 5 min bellows (Bhastrika) followed by Om Chant, 10 min SK, and 5 min ANBC: Seated w/eyes closed and gentle attention on breath |
| * Sureka, 2015 [103]India, prison hospital | RCTMale inmates w/substance dependence | Ix: 39.3 y, 0%, 55C: 38.8 y, 0%, 56 | For 6 wks, 23 m/day:Ix: Guided SKY breathing: 8 min Ujjayi, 5 min bellows (Bhastrika), and 10 min SKC: Sitting, eyes closed, attention to breath |
| Thomas, 2017 [50] UK, general practices | RCTAdults w/impaired asthma control | Ix-1: 56 y, 63%, 261Ix-2: 55 y, 69%, 132C: 57 y, 63%, 262 | Ix: Guided breath training delivered either via DVD (Ix-1) or 3 biweekly 30–40 min sessions w/physiotherapist (Ix-2), comprising: nose breathing, abdominal breathing, slow breathing, controlled breath holding, relaxationC: Usual care |
| * Fiskin, 2018 [104]Turkey, medical center | RCTPregnant women w/GDM | Ix: 30.6 y, 100%, 30C:31.3 y, 100%, 30 | 30 days of:Ix: Unguided diaphragmatic breathing, 5 min in the morning before leaving bed C: Standard care, invited to talk about their pregnancies 2×/mo |
| * Watson, 2022 [105]New Zealand hospital | RCT (cross-over)Postmenopausal women w/stress cardiomyopathy | 66.8 y (2.7)Ix: 100%, 12C: 100%, 12 | One 3 min session each, separated by 5 days, of guided:Ix-1: Hyperventilation; Ix-2: Slow breathing w/3 s hold |
| F. Simulated Stress | |||
| Simulated Stress Populations: Stress/anxiety as primary outcome | |||
| Kamath, 2017 [44] India, medical school | RCTHealthy, yoga-naive undergrad medical students | Range 19–24 yIx: 60%, 15C: 80%, 15 | After brief instruction and practice for 15 min prior to simulated public speaking task:Ix: Guided ANBC: Sitting in quiet room |
| Simulated Stress Populations: Stress/anxiety as secondary outcome | |||
| Holmes, 1978 [42] US, university setting | 3-arm clinical trial (randomization/Tx allocation unclear)Undergraduate students | Age not reported, 54%, 111 | 90 s threat of electric shock (never administered) or visual stimulation, after 5 min of:Ix-1: Respiration tracing, breathing at pace where real-time respiration polygraph pen matched previously recorded resting respiration polygraphIx-2: Attention tracing, using hand-held knob to follow previously recorded resting respiration polygraph recordingC: Sitting quietly and relaxing |
| * McCaul, 1979 [106] US, university setting | RCT, 3 × 2 + 1 factorial-designedMale undergraduate students | Ix-1: 0%, 29 Ix-2: 0%, 28 Ix-3: 0%, 28C: 0%, 14 (Ages not Reported) | 3 groups threatened w/impending electric shock, w/possibly 2 min (duration unclear) of unguided: Ix-1: Slow-pace regulated breathing (8 bpm); Ix-2: Normal-pace regulated breathing (16 bpm); Ix-3: Non-regulated breathingC: No breath pacing, no expectations, no threat, anticipation of visual stimulationIx-1 and Ix-2 had 2 min practice before threat |
| Sakakibara, 1996 [48] Japan, university setting | RCTHealthy college students | 23 y Ix-1: 40%, 10 Ix-2: 30%, 10 C: 50%, 10 | Prior to 2 min threat (electric shock) anticipation w/15 bpm paced breathing, 5 min of unguided:Ix-1: Slow breathing (8 bpm) Ix-2: **Fast breathing (30 bpm)**C: Spontaneous breathing |
| * Telles, 2019 [49]India, residential yoga center | Randomized cross-over studyAdult males w/yoga breathing experience | 28.4 y (8.2), 0%, 50 | In 3 separate, 18 min sessions across 3 days, w/timed shape and size discrimination task before/after each: Ix-1: ANBIx-2: Breath awarenessC: Quiet sitting |
| Meier, 2020 [46]Canada, setting unclear | RCTYoung healthy men and women | 23.8 y (4.5), 51%, 35Ix-1: 12Ix-2: 11C: 12 | 30 m, prior to simulated stress test (modified TSST-G):Ix-1: Guided relaxation breathing while standing Ix-2: Laughter yogaC: Reading magazines or books |
| * Sharma, 2022 [107]India, university setting | Randomized cross-over studyUniversity males w/yoga breathing experience | 24 y (4), 0%, 38 | 6 breath sessions in random order, w/timed SLCT before/after each, for 18 min w/1 min rest every 5 m, guided:Ix-1: ANB; Ix-2: Bellows breathing; Ix-3: Bumblebee breathing; Ix-4: High-frequency breathing at 50–65 bpm; Ix-5: Breath awareness; Ix-6: Quiet seated rest |
Twenty-five of the 58 studies were conducted in Asia; 14 in Europe; 13 in the United States; 1 in Canada; 2 in Brazil; 2 in North Africa; and 1 in New Zealand. Twenty-nine studies were based in hospital or clinical care settings, such as primary care, medical centers, or other clinical settings. Fifteen were at university settings, including medical schools. Three were based at yoga centers, 2 at elementary or secondary schools, 1 in an automotive assembly plant, and 1 was remote. The remaining 6 settings were unclear.
Within the 58 studies, 72 breathing interventions that met our inclusion criteria were identified, and 54 such interventions significantly reduced participants’ stress/anxiety. The most common type of breathing intervention was general slow breathing, which was included in 36 of the studies. The second most common selection was deep diaphragmatic breathing (20), followed by fast breathing (14), alternate or unilateral nostril breathing (ANB, UNB; 11), breathing with holds/pauses (7), extended exhale breathing (6), normal-rate breathing (2), and paced or pursed lip breathing (5).
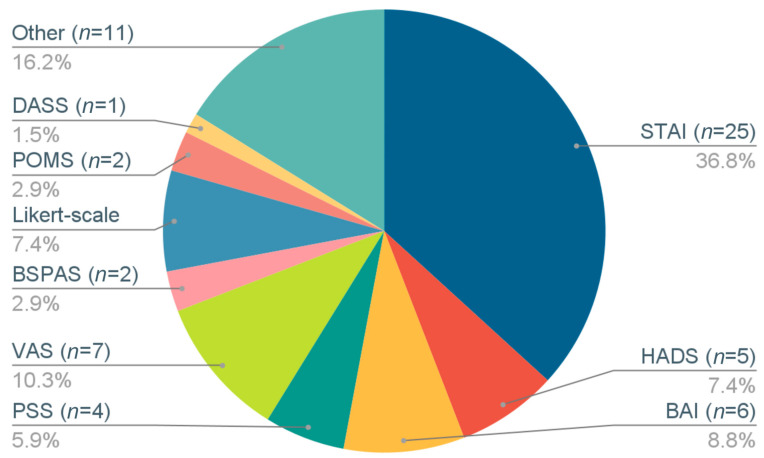
The following psychometric measures of stress and anxiety were used (Figure 2): State Trait Anxiety Inventory (STAI) in 25 studies; Visual Analog Scales (VAS) in 7; Beck Anxiety Inventory (BAI) in 6; Hospital Anxiety and Depression Scale (HADS) in 5; stress/anxiety Likert scales in 5; Perceived Stress Scale (PSS) in 4; Profile of Mood States (POMS) in 2; Burn-Specific Pain Anxiety Scale (BSPAS); Depression Anxiety Stress Scales (DASS) in 1; and other stress/anxiety metrics in 11. Additional metrics were included in some studies to evaluate the impact of breathing interventions on cognition, lung function, physiological stress, psychological well-being, and other health-related conditions.
Figure 2.
Distribution of stress/anxiety outcome metrics used in included studies. Rounding errors result in whole-number percentages summing to more than 100%. Total number of studies using metrics sums to more than 58 because some studies incorporated more than one outcome metric. STAI, State-Trait Anxiety Inventory; HADS, Hospital Anxiety and Depression Scale; BAI, Beck Anxiety Inventory; PSS, Perceived Stress Scale; POMS, Profile of Mood States; BSPAS, Burn-Specific Pain Anxiety Scale; DASS, Depression Anxiety Stress Scale; VAS, Visual Analog Scale.
3.2. Study Characteristics and Outcomes by Population
Table A2 describes the stress/anxiety metrics, outcomes, and quality scores of included studies by population.
3.2.1. Youth
Four studies were conducted among youth, of which all were RCTs, and three examined stress or anxiety as a primary outcome. Sample sizes ranged from 27 to 154, mean age from 8 to 15 years, and intervention duration from 1 to 45 days. Of the four interventions in these studies, three used slow breathing and one included a mix of slow breathing, fast breathing, and ANB. All four yielded significant stress/anxiety benefits.
3.2.2. Healthy Adults
Fourteen studies were conducted among healthy adults, of which eight were RCTs and eight examined stress or anxiety as a primary outcome. Sample sizes ranged from 16 to 761, age from 17 to 70 years, and intervention duration from 1 to 273 days. Of the 21 interventions in these studies, 13 used slow breathing, 1 used regular-paced breathing, 1 used cyclic hyperventilation with holds, 1 used fast pranayama, 1 used slow pranayama, 1 used slow and fast pranayama, 2 used ANB, and 1 included a mix of slow breathing and ANB. Seventeen interventions yielded significant stress/anxiety benefits.
3.2.3. High-Anxiety Populations
Four studies were conducted among high-anxiety populations, of which two were RCTs and three examined stress or anxiety as a primary outcome. Sample sizes ranged from 18 to 46, mean age from 24 to 43 years, and intervention duration from 2 to 56 days. All four interventions in these studies incorporated some type of deep, diaphragmatic and/or slow breathing, and all yielded significant stress/anxiety benefits.
3.2.4. Clinical Populations, Acute
Of the 20 studies conducted in clinical settings among populations with acute clinical conditions, 15 were RCTs and 16 examined stress or anxiety as a primary outcome. Sample sizes ranged from 20 to 272, mean age from 21 to 75 years, and intervention duration from 1 to 56 days. Of the 21 interventions in these studies, one used ANB, three included a mix of slow and fast breathing, and the rest used slow breathing. Seventeen interventions yielded significant stress/anxiety benefits.
3.2.5. Clinical Populations, Chronic
Of the nine studies conducted in clinical settings among populations with chronic health conditions, six were RCTs and three examined stress or anxiety as a primary outcome. Sample sizes ranged from 11 to 655, mean age from 31 to 67 years, and intervention duration from 1 to 182 days. Of the eleven interventions used within these studies, six used slow breathing, one used fast breathing, one used UNB, and three included a mix of fast and slow breathing. Seven interventions yielded significant stress/anxiety benefits.
3.2.6. Simulated Stress Settings
Of the seven studies conducted among populations undergoing simulated stress situations, five were RCTs and three examined stress or anxiety as a primary outcome. Sample sizes ranged from 30 to 111, mean age ranged from 23 to 28 years among studies reporting mean data and from 19 to 24 among studies not reporting means, and intervention duration for all was one day. Of the eleven interventions in these studies, six used slow breathing, one used regular-paced breathing, one used fast breathing, one used fast pranayama, and two used ANB. Five interventions yielded significant stress/anxiety benefits.
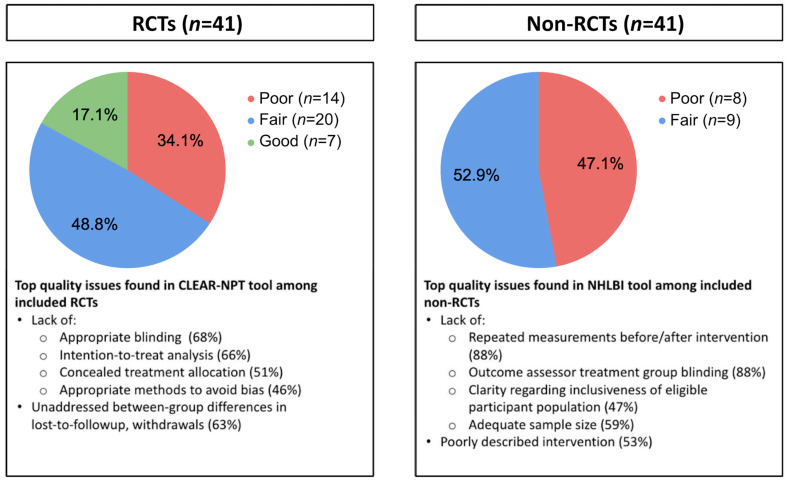
3.3. Study and Reporting Quality
Of the 58 included studies, 7 RCTs and 0 non-RCTs received “good” quality ratings, 20 RCTs and 9 non-RCTs received “fair” ratings, and 14 RCTs and 8 non-RCTs received “poor” ratings (Table A2). Figure 3 summarizes the top identified quality issues. Among RCTs, the main concerns related to the absence of the following: blinding, intention-to-treat analysis, concealed treatment allocation, and analytic consideration of between-group differences in lost-to-follow-up and withdrawals. Among non-RCTs, the main concerns related to the absence of the following: repeated measurements, assessor blinding, population inclusiveness, adequate sample size, and intervention description in the report. The reviewers noted other common quality issues during abstraction. Many pre-post studies did not include a control group, and those that did lacked an active control. Several intervention descriptions were vague and lacked detail about factors such as breath rate, bpm, and instructions for breath practices. Adherence and compliance with at-home practice were measured and reported poorly or not at all, and reports on human-guided training lacked detail in duration, timeline, and extent of guidance.
Figure 3.
Top quality issues in 58 studies included in review by study type. Quality score calculated by dividing number of met criteria by number of applied criteria and normalizing to 0–1.0 range. Quality score categories defined as follows: <0.6, poor; 0.6–0.8, fair; and >0.80, good. CLEAR-NPT, checklist to evaluate a report of a nonpharmacological trial; NHLBI, National Heart, Lung, and Blood Institute; RCT, randomized controlled trial.
3.4. Synthesis Results
3.4.1. Study Design Parameters
The following study design parameters were identified for analysis regarding their association with intervention effectiveness: study type (RCT, non-RCT); stress/anxiety as the primary or secondary outcome; control group type (usual care/no intervention, breath practice + other components, such as yoga or meditation, non-breath active control, meditation or mindfulness, no control); and the number of stress/anxiety outcome measurements (1, 2, 3+).
None of the study design parameters were significantly associated with intervention effectiveness (Table 2). RCTs made up 72% of effective (39 of 54) and 67% of ineffective (12 of 18) interventions, with these proportions not differing significantly (p = 0.78). Stress/anxiety was a primary outcome in 67% of effective and 61% of ineffective interventions (p = 0.67). There was no association between effectiveness and use of control group (p = 0.19). Stress/anxiety was measured as follows: once in 4% (2 of 54) of effective and 6% (1 of 18) of ineffective interventions; twice, once before and once after the intervention, in 54% and 67% of the respective interventions; and three or more times in 43% and 28%, respectively (p = 0.43).
Table 2.
Association of intervention parameters with intervention stress/anxiety reduction effectiveness among 72 included interventions.
| Parameter | N | _p_-Value |
|---|---|---|
| Study design | ||
| Study design (RCT vs. other) | 72 | 0.77 |
| Control group (yes/no) | 72 | 0.19 |
| Primary vs. Secondary stress/anxiety outcome metric | 72 | 0.78 |
| # stress/anxiety outcome measurements (1, 2, 3+) | 72 | 0.43 |
| Breath practice and implementation | ||
| Population | 72 | 0.14 |
| Breath practice pace | 72 | 0.01 * |
| Breath pace other than fast-only | 70 | 0.10 |
| Human-guided training (yes/no) | 72 | <0.001 * |
| # human-guided sessions (0, 1, 2−3, 4+) | 71 | <0.001 * |
| Human-guided training included within device-guided interventions (yes/no) | 15 | 0.03 * |
| Single vs. Multiple sessions | 72 | <0.001 * |
| Long-term vs. No long-term practice | 72 | 0.005 * |
| Intervention duration (1, 2−5, 6−30, or 31+ days) | 71 | <0.001 * |
| Session duration: | ||
| <5, 5–10, 11–20, or >20 m | 61 | 0.03 * |
| <5 or ≥5 m | 61 | 0.005 * |
| Among sessions ≥5 m:5–10, 11–20, or >20 m | 52 | 0.81 |
| Group vs. individual practice vs. both | 67 | 0.79 |
| At-home practice (yes/no) | 72 | 0.24 |
3.4.2. Breathing Practice and Implementation Parameters
The following breath practice and intervention implementation parameters were identified for analysis: population category; type and pace of breath practice (slow-only, fast-only, a combination of fast and slow with or without ANB/UNB, regular pace, ANB/UNB alone); the use of human-guided training (yes/no); number of human-guided sessions (0, 1, 2–3, 4+); among device-guided interventions, use of human-guided training (yes/no); number of sessions (single or multiple); long-term practice (≥6 sessions over ≥1 week, yes/no); intervention duration (1, 2–5, 6–30, or 31+ days); session duration (<5, 5–10, 11–20, or >20 min); group- or individual-only or group + individual practice; and inclusion of at-home individual practice (yes/no).
Intervention effectiveness was not significantly associated with population category (p = 0.14), session duration beyond 5 min (p = 0.81), group versus individual versus group + individual practice (p = 0.79), and use of at-home practice (p = 0.24; Table 3). Among youth and high-anxiety adults, all interventions were effective. Among healthy adults, 17 of 21 (81%) interventions were effective; these numbers were 17 of 21 (81%) among acute clinical populations, 7 of 11 (64%) among chronic clinical populations, and 5 of 11 (45%) among simulated stress populations. The majority of interventions were individual-only (61% of effective and 56% of ineffective interventions) or group-only (28% and 33%, respectively), with 6% of effective and 0% of ineffective interventions including both group and individual sessions. Five interventions were unclear about this parameter in the published paper. Session durations ranged from 1.3 to 60 min for effective and 1–45 min for ineffective interventions. Six percent of effective and 33% of ineffective intervention session durations were <5 min, and 80% of effective and 50% of ineffective interventions’ sessions lasted ≥5 min; duration was unspecified in the remaining 11 interventions. Among 5 min or longer interventions, 30% of effective and 17% of ineffective ones were 5–10 min, 19% of effective and 17% of ineffective ones were 11–20 min, and 31% of effective and 17% of ineffective ones were >20 min. Six percent of effective and zero ineffective interventions were combined group and individual, and 6% of effective and 11% of ineffective interventions did not report group versus individual implementation. At-home practice was included in 35% of effective and 17% of ineffective interventions.
Table 3.
Outlier interventions with explanatory practice and research caveats.
| Intervention | Potential Caveats | ||
|---|---|---|---|
| Author, Year (Population) | Description | Practice | Research |
| Effective practices incorporating zero core components | |||
| Schlatter, 2022 [79](healthy adults) | 5 min of 6 bpm relaxing breathing prior to simulated medical scenario and test | Investigator present during device-guided practice Physiologically trained residents | |
| Ineffective practices that incorporated the following components: | |||
| Human-guided training | |||
| Meier, 2020 [46] (simulated stress) | 30 min guided standing relaxation breathing prior to simulated stress test | Standing still Interrupted practice | |
| Human-guided training, multiple sessions | |||
| Kamath, 2017 [44](simulated stress) | 15 min guided ANB prior to simulated public speaking task | Highly technical practice ANB task performance effects may attenuate stress-reducing effects | |
| Ratcliff, 2019 [47](clinical, acute) | Guided diaphragmatic breathing for 10 min prior to and during breast biopsy | Difficult to execute lying on stomach and remaining still for biopsy | Practice guides not blinded |
| Human-guided training, multiple sessions, long-term practice | |||
| Thomas, 2017 [50](clinical, chronic) | Guided diaphragmatic breathing with breath holds, guided via DVD | Practices not continued through outcome period Outcomes reported at baseline and 12 mo | |
| Same as above, delivered by physiotherapist |
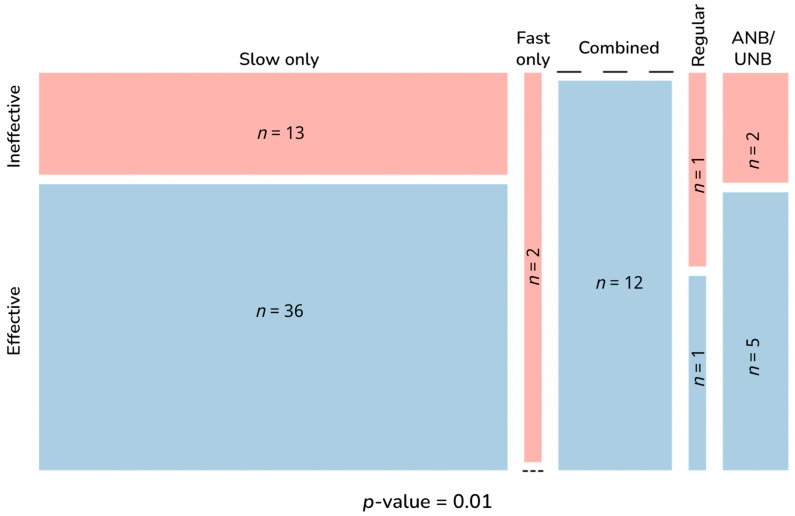
Intervention effectiveness was significantly associated with the following parameters: breath pace (p = 0.01); use of human-guided training (p < 0.001); number of human-guided sessions (p < 0.001); among device-guided interventions, use of human-guided training (p = 0.025); number of sessions (p < 0.001); long-term practice (p = 0.005); intervention duration (p < 0.001); and session duration <5 versus ≥5 min (p = 0.005). Effectiveness was no longer associated with breath pace when considering any pace besides fast-only (p = 0.10) or when comparing fast-only to any other pace (p = 0.06). All three human-guided training parameters were subsumed into the broader category of human-guided training, as were all three intervention duration measures into long-term practice. Thus, five main parameters were identified as components associated with stress reduction effectiveness, which were subdivided into exclusionary and core components. Breath pace and session duration comprised exclusionary components, in which avoiding fast-only practices and sessions <5 min appears conducive to but not sufficient for intervention effectiveness, while incorporating the core components of human-guided training, multiple sessions, and long-term practice actively contributes to stress reduction benefits.
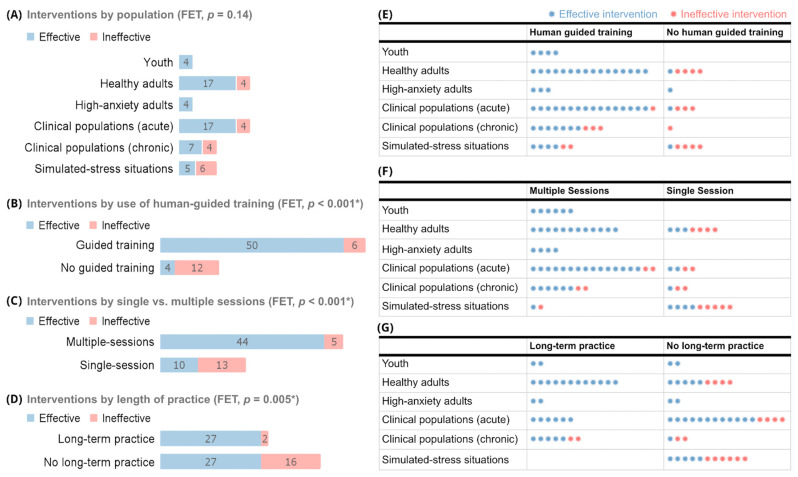
Figure 4 shows the numbers of effective and ineffective interventions by breath pace. Figure 5 shows how effective and ineffective interventions were distributed by population (Figure 5A) and by use of human-guided training, multiple sessions, and long-term practice, both overall (Figure 5B–D) and by population (Figure 5E–G).
Figure 4.
Effective and ineffective interventions by breath practice pace. Blue shaded portion in each category column represents the proportion of effective interventions; red shaded portions reflect ineffective interventions. Dashes represent the lack of effective interventions in the fast only category and ineffective interventions in the combined category. _p_-value calculated with Fisher’s exact test. Breath intervention types defined as fast, breath pace >20 bpm; slow, <12 bpm; regular, 12–20 bpm; combined, fast, and slow breathing with or without ANB/UNB; and ANB/UNB. ANB, alternate-nostril breathing; n, number of interventions; UNB, unilateral nostril breathing.
Figure 5.
(A–G) Effective and ineffective breath interventions by population and use of human-guided training, single or multiple sessions, and long-term practice. Each panel is collectively exhaustive of all 72 interventions from 58 studies in total. Numbers in bar graphs of panels (A–D) represent numbers of interventions. Each colored asterisk in panels (E–G) represents one study. Blue bars and asterisks represent effective interventions; pink ones represent ineffective interventions. Human-guided training defined as use of live or pre-recorded audio or video human instruction throughout at least the initial breath session. Multiple sessions defined as performing breathing practice more than once. Long-term practice defined as performing ≥6 sessions over ≥1 week. (A,E–G) Interventions were divided into six broad population categories: youth (n = 4); healthy adults (n = 21); high-anxiety adults (n = 4); clinical, chronic (n = 11); clinical, acute (n = 21); and individuals placed in simulated-stress situations (n = 11). FET, Fisher’s Exact Test. * Statistically significant at p < 0.05.
Overall, 44 of the 54 effective and 10 of the 18 ineffective interventions incorporated some form of human-guided training during participants’ initial breath practice session (Figure 5B), with a total number of human-guided sessions ranging from 1 to 40. These sessions ranged from 3 to 60 min among effective interventions and from 3 to 45 min among ineffective ones. Fifty of the 54 effective and 1 of the 18 ineffective interventions incorporated multiple breath practice sessions throughout the intervention (Figure 5C), with up to 100+ sessions incorporated. Long-term practice was incorporated in 27 of the effective and 2 of the ineffective interventions (Figure 5D), ranging from 1 week to 3 months of practice.
Considering each population specifically, interventions in youth and high-anxiety adults were always effective (Figure 5A,E). All eight of these interventions incorporated at least one of the three core components of human-guided training, multiple sessions, and long-term practice; and none used fast-only or <5 min practices. In healthy adults, all seventeen effective interventions incorporated at least one core component, and all four ineffective ones used zero core components and <5 min sessions. The effective interventions in clinical and simulated-stress populations always used at least one core component; three used <5 min sessions. Among the fourteen ineffective interventions within those populations, seven used one or more core components; of these seven, one used <5 min sessions, and another used both fast-only and <5 min practices.
Synthesis outliers were defined as effective interventions that did not incorporate any of the three core components or ineffective interventions that avoided both fast-only breathing and sessions <5 min and incorporated at least one core component. Six outlier interventions were identified and explored to determine explanatory variables that may be considered potential model caveats (Table 3). One intervention was effective despite not incorporating human-guided training, multiple sessions, or long-term practice [79]. Effectiveness may relate to the investigator’s presence throughout the device-guided practice, which may have led to unplanned human guidance that was not reflected in the study report. It is also possible that the highly educated and physiologically trained 5th-year medical residents who participated in this study easily adapted to learning a breath practice.
Five interventions were ineffective despite avoiding fast-only and <5 min practices and incorporating at least one core component (Table 3). In Meier (2020), the fact that participants were standing still for a 30 min practice and were repeatedly interrupted for stress surveys likely explains the lack of effectiveness. The ANB intervention in Kamath (2017) was ineffective despite incorporating both human-guided training and multiple sessions. Of the three other ANB interventions used in simulated stress situations, the one effective intervention [107] incorporated human-guided training among a population with prior yoga breathing experience [107]. The two ineffective ones had either human-guided training in a yoga-naive population [44] or no such training in a yoga-breathing-experienced population [49]. All other ANB studies were effective in reducing stress and incorporated ≥2 of the three core components. It may be that in a highly technical practice such as ANB, multiple sessions or prior experience plus human-guided training are required for stress-reducing effects.
In Ratcliff (2019), effectiveness may have been diluted by the difficulty of doing diaphragmatic breathing while lying still and face-down on a procedure table during a breast biopsy. Investigator bias may also have influenced effectiveness, with unblinded investigators leading this active control practice. In Thomas (2017), the practice was not continued by many participants for the entire 12-month measurement window.
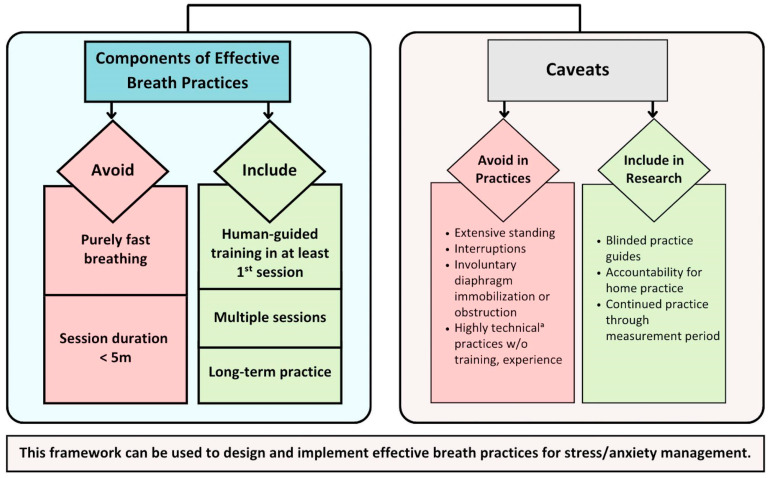
3.4.3. Framework Development
Synthesis results were used to develop a conceptual framework of components associated with effective stress reduction breathing interventions (Figure 6): avoiding fast-only breathing and session durations <5 min; and including human-guided training, multiple sessions, and/or long-term practice. The framework also identifies caveats reflecting factors that may potentially reduce intervention effectiveness, such as extensive standing, repeated interruptions, involuntarily restricted diaphragmatic expansion, and inadequate training for highly technical practices. Intervention effectiveness may also be impeded in studies that lack blinded practice guides, accountability for at-home practices, and practice continued throughout measurement periods.
Figure 6.
Conceptual framework of effective stress reduction breath practices. Based on analysis of 58 studies with 72 total interventions. Components in left-hand box were significantly associated with effectiveness. Caveats describe factors that may impact intervention effectiveness regardless of component inclusion. Human-guided training defined as use of live or pre-recorded audio or video human instruction throughout at least the initial breath session. Multiple sessions defined as performing breathing practice more than once. Long-term practice defined as performing ≥6 sessions over ≥1 week. a Highly technical includes breath pace far from regular pace, e.g., extremely fast or extremely slow, or ANB, UNB. ANB, alternate-nostril breathing; UNB, unilateral nostril breathing; w/o, without.
4. Discussion
4.1. Overview
We reviewed the peer-reviewed published literature to evaluate practice components associated with stress reduction effectiveness. We identified 58 clinical studies, comprising 41 RCTs and 17 single-arm, pre-post, non-randomized trials; 5407 total participants were included, with a mean age of 35.9 years and 43.92% female. Among the 72 included interventions, breathing practices significantly reduced stress/anxiety in 54 interventions. Findings were used to develop a conceptual framework of breath practice effectiveness, specifying that stress-reducing interventions incorporated five components: avoidance of fast-only breathing and sessions <5 min; and inclusion of human-guided training, multiple sessions, and/or long-term practice. Neither the population doing the practice, other breath paces besides fast-only, session duration beyond 5 min, group versus individual practice, nor setting were associated with effectiveness.
Breathing practices have become an increasingly popular stress reduction tool with widespread benefits for mental health. A simple PubMed search of “breathing practices” yields 18,721 results, over half of which have been published since 2010. Mobile applications are bringing breathwork to broader audiences, with the number of existing breath apps in the double digits. Despite this popularity, not everyone is aware of the benefits of breathwork; among those who are, emphasis is often placed on specific practice types and durations. Though many breath coaches pride themselves in providing the “best” practice, no singular effective protocol has been established and supported by scientific analyses.
Previous reviews have provided insight into the psychological and/or physiological benefits of slow breathing [52], diaphragmatic breathing [36], ANB [38], and other breath practices [23,25,35,37]. While these studies have examined a range of populations, their selection criteria have precluded more nuanced analyses. Although the analysis by Fincham and colleagues attempted to investigate subgroup effects, they included only 12 studies [35]. In addition, most reviews include breathing interventions incorporating biofeedback [23,25,35,52], which is not readily applicable to at-home practice. Ours is the first work of its kind to disentangle among varied populations the components of isolated breathing interventions that contribute to stress and anxiety reduction from those that do not. The framework provides clear, specific recommendations for individuals implementing their own practices, professionals developing and leading stress reduction programs, and researchers studying the effects of breath practices on stress outcomes.
4.2. Exclusionary Components
4.2.1. Breath Pace
Intervention effectiveness was less likely during fast-only breathing versus any other pace, consistent with other reviews finding benefits associated with slow-paced breathing practices [23,35]. Polyvagal [108] and neurovisceral integration [109] theories favor slow over fast breathing due to its activation of the parasympathetic nervous system and accompanying effects on the mind and brain [52]. Our analysis further suggests that, provided fast-only practices are avoided, specific breath pace may not matter, as effectiveness was not associated with pace when fast-only practices were excluded.
Interestingly, fast breathing can successfully reduce stress when paired with slower paces or holds, as seen in the 12 fast + slow interventions, all of which were effective. Such practices may provide benefits by teaching practitioners to slow their breathing patterns and self-soothe during stressful situations; or by building CO2 tolerance, which is inversely related to symptoms of stress and anxiety [110]. They may, alternatively, train the autonomic nervous system to engage the parasympathetic branch more readily following high sympathetic activation [111]. Yet another interpretation resides in the connection between breathing patterns and the brain’s neural circuitry, whereby shifting breathing patterns may correspondingly shift neural circuit dynamics and thus mood and mental state [26].
If this explanation holds true, regular-paced breathing practices would provide no such benefit. Because only two interventions met our 12–20 bpm regular-paced breathing definition, we cannot definitively assess the stress-reducing effects of regulated breathing at one’s normal pace. Of the two regular-paced interventions, one was ineffective, which may relate to its lack of framework adherence [42]. The other was effective, possibly due to human-guided training and its 12 bpm pace, which may have been slow enough to engender benefits [78].
The lack of effectiveness observed among fast-only breathing interventions should be interpreted with caution, given their underrepresentation in our analyses. The two fast-only interventions either lacked framework adherence [48] or incorporated poor study design [105]. Should breathwork’s benefits relate to respiration-induced neural entrainment, any breathing pace different from one’s usual pattern may offer stress relief—even fast-only practices. Fast breathing could hypothetically reduce stress by acting as an acute stressor that builds resilience over time or by providing a sense of control from learning to regulate one’s own breathing and internal state.
4.2.2. Session Duration
The significant association between intervention effectiveness and session duration was driven by interventions using sessions <5 min, six of nine of which were ineffective. Among these, however, four incorporated no core component, and the remaining two included other factors that may have influenced effectiveness. Regarding the latter, stress reduction may have also been hindered by fast-only breathing in Watson and colleague’s intervention and by the discomfort of doing deep diaphragmatic breathing after abdominal surgery in Aktas and colleagues’ intervention [39,105]. The presence of core components in the three effective interventions [39,105,106] suggests that such short sessions may be particularly sensitive to practice implementation, such that human guidance, multiple sessions, or long-term practice are required for effective stress reduction.
The lack of significant association between effectiveness and session durations ≥5 min indicates that any session duration beyond 5 min can be effective. While no other studies have assessed differences in breath practice effectiveness due to session length, many generally assume a dose-response relationship in which longer sessions are inherently better. Our findings indicate that simply engaging in a breathing practice provides benefits, with sessions as short as 5 min yielding comparable benefits to longer sessions. Interestingly, a recent review by Strohmaier and colleagues reported similar findings about mindfulness-based programs, with larger doses of mindfulness practices no more effective than smaller ones [112].
4.3. Core Components
4.3.1. Human-Guided Training
The association between human-guided training during initial sessions and effectiveness highlights the value of proper breath mechanics, diaphragmatic engagement, and the ability to control breath pace. Hearing real-time instructions allows participants to receive performance cues and, in the case of live guidance, to ask questions and/or get feedback. Such guidance may enhance adherence and stress reduction outcomes. The fact that this training could be live or pre-recorded and as minimal as one 5 min session implies accessibility to broad populations, although it is notable that all effective interventions among high-anxiety populations included two or more human-guided sessions.
While important, human-guided training does not guarantee stress reduction benefits, and the lack thereof does not prevent effectiveness. Effectiveness may be compromised despite human guidance in the presence of clinical-related breathing restrictions, fast-only or highly technical breathing techniques, and prolonged latency between intervention and outcome measurements. More human guidance may be needed in situations of clinical stress, such as undergoing a breast biopsy [47], for learning more technical practices, such as ANB and UNB, or for high-anxiety populations. In some cases, lack of human-guided training may be made up for by multiple sessions that allow participants to learn these nuances themselves over time. Of the four effective interventions that lacked such training, three included multiple sessions [39,80,106], and the other [79] may have incorporated some unreported and unintentional human guidance due to the investigator’s presence during the practice.
4.3.2. Multiple Sessions
The observed importance of multiple sessions likely relates to greater practice familiarity and consistency. Multiple sessions took various forms, ranging from two guided individual sessions in one day [67] to five group sessions over 9 months alongside twice-daily at-home practice [71]. Intervention effectiveness persisted despite such heterogeneity.
Although 41 of the 44 effective multi-session interventions included at least one human-guided session, having multiple sessions alone appears to support breath practice effectiveness. Three of the four effective unguided interventions included multiple sessions [39,80,106]; and significant associations between effectiveness, intervention duration, and number of sessions suggest that breathing practices are more beneficial when performed repeatedly. Nevertheless, some single-session interventions were, in fact, effective, with 10 of 23 providing stress reduction benefits. Nine of these 10 were human-guided, suggesting that adequate training may, in some cases, make up for multiple sessions in breath practice efficacy.
Examining the four multi-session interventions that lacked human guidance also suggests that interventions without human-guided training are more likely to be effective when they include simple, easy-to-follow breathing practices alongside device guidance. Interestingly, seven of eight single-session interventions with such simple, device-guided practices were ineffective, highlighting the importance of multiple sessions when human guidance is lacking.
4.3.3. Long-Term Practice
Practicing for a minimum of six sessions over at least a week was significantly associated with effectiveness, suggesting that breathing practices are more likely to confer stress-reducing benefits when performed regularly over time. As with many behaviors, consistent practice is often key to effective skill learning and adaptation. Although these results contrast with other findings of no treatment duration effect [24,35], those reviews may have been limited by small samples, study heterogeneity, and exclusion of human-guided training, which may interact with long-term practice.
All long-term interventions in our review also incorporated human-guided training, suggesting that human-guided training may underlie the association we found between long-term practice and effectiveness. However, examining the relationship further suggests otherwise: 6 of the 27 effective interventions with long-term practice had only one human-guided session, with the remaining sessions performed at home, unguided. If human-guided training were solely responsible for intervention effectiveness, these interventions likely would not have demonstrated benefits, as their outcome measures were taken up to 45 days after human-guided training [20]. It is more likely that long-term practice enhances intervention effectiveness when coupled with at least one human-guided session, which may help teach novice practitioners proper technique.
4.4. Components Not Associated with Effectiveness
Our analysis found no association between effectiveness and study population. Because breathing acts directly on the autonomic nervous system as a “bottom-up” approach to stress reduction [113,114,115,116,117,118], its benefits likely persist across diverse populations. This finding contrasts with those from Fincham and colleagues, whose review reported a significant benefit of breathwork on stress in non-clinical populations only [35]. However, their analysis may have been underpowered with only one intervention in mental health populations; and another recent meta-analysis found significant effects in both psychiatric and healthy populations [24].
It Is worth noting that all interventions in youth and high-anxiety populations were effective. Perhaps this is a coincidence due to the small sample sizes of four interventions each or due to the prevalence of human-guided training and multiple sessions in these interventions. These two core components may alone be a golden ticket to effective stress reduction with breathing practices. Of the 45 interventions in any population that incorporated both of these components, only four were ineffective [44,47,50], but this was likely due to taxing clinical situations [47], inadequate training or experience for a more technical practice [44], and practice discontinuation [50]. Alternatively, the effects of breathwork may be more potent in high-anxiety populations and youth. The respiratory dysregulation and high chronic stress accompanying anxiety disorders may augment sensitivity to breath practice interventions [53]. Accustomed to being in learning environments, youth may easily catch on to these practices with human guidance, and their developing nervous systems may be highly receptive to interventions [119].
We found that interventions given to groups were equally effective as those given to individuals, a finding corroborated by other recent literature [35]. However, given the small number of group interventions in high-anxiety and acute chronic populations, it is possible that populations with particularly dysregulated breathing [28,29,33] may fare better with one-on-one attention.
Interventions with multiple at-home practice sessions were equally as effective as those with multiple sessions administered via human guidance. Thus, human-guided training does not appear necessary beyond the first session, after which breathing practices can be carried out individually at home. Nonetheless, interventions incorporating at-home practices should motivate and assess practice compliance, as our three ineffective interventions with at-home practice [39,50] reported either inconsistent or unknown practice compliance.
4.5. Framework Caveats
By scrutinizing the six outlier interventions, we identified framework caveats that may either improve or undermine effectiveness, independent of the proposed framework components. Regarding the former, our analysis indicates that even breathing practices that diverge slightly more from one’s spontaneous pattern may not require human-guided training, given adequate device guidance paired with strong accountability and physiologically trained participants. While Schlatter and colleagues’ effective intervention [79] lacked human guidance, their 6 bpm practice among 5th-year medical residents was guided via a screen cursor with an investigator present to ensure practice adherence.
On the physical side, breath practices should be performed in a comfortable position without prolonged standing or involuntary diaphragmatic immobilization. The 30 min of standing during Meier and colleagues’ human-guided intervention may have caused discomfort and interfered with potential benefits [46]; though perhaps shorter standing practice durations would be effective. Deep or diaphragmatic breathing may provide minimal benefit when voluntary full diaphragmatic movement is lacking, such as during a breast biopsy [47]. Practices placing less emphasis on the diaphragm and more emphasis on breath cadence, such as extended exhale or general slow breathing, may offer better relief in such situations.
Breath practices may also be less likely to reduce stress when they include interruptions. While practicing breathing in a standing position may have influenced Meier’s findings, so could its repeated data collection interruptions, which may have reduced focus and induced sufficient stress to negate benefits.
Analysis of included ANB/UNB interventions suggests that more technical practices may require extra training or experience. One ANB intervention was ineffective despite fitting our framework, incorporating a single human-guided session among yoga-naive participants [44]. Of our four effective ANB/UNB interventions carried out in novice practitioners, all included human-guided training for a minimum of four sessions [69,77,88,100]. These findings are consistent with ANB’s stress-reducing effects reported elsewhere, in which all included interventions incorporated long-term practice alongside human-guided training [38]. Nonetheless, an effective human-guided but short-term intervention among individuals with prior ANB or yoga breathing experience suggests that single-session ANB may confer benefits when carried out among experienced practitioners and with human-guided training [107]. The technical nature of learning and practicing ANB may initially attenuate its stress-reducing effects, specifically for inexperienced practitioners, until repeated practice occurs. Conversely, human-guided training and long-term practice may not be necessary for exceedingly simple breathing practices, such as those at a slightly slower-than-normal pace (e.g., 10 or 8 bpm) that may be more accessible to breathwork-naive individuals [80,106].
Our findings also suggest that long-term effectiveness requires long-term compliance. Participants in Thomas and colleagues’ 2017 ineffective interventions engaged in regular practice for 6 to 8 weeks but were not practicing most days by the time of the 12-month stress measurement [50]. Another review found that breathwork benefits may persist for a brief period after practice ends, although practice adherence was not assessed in that study [35]. In our analysis, all other studies with long-term measurements included either regular guided practice throughout or specific instructions and motivation for at-home practice.
4.6. Limitations and Future Directions
Findings from this analysis must be considered in light of its potential limitations. Due to the mediocre-at-best methodological quality of included studies, some effectiveness findings may not be as reliable or valid as assumed. Future research should reduce potential bias by incorporating appropriate blinding and measurements, intention-to-treat analyses, concealed treatment allocation, and larger sample sizes. The articles included in our analysis demonstrated wide heterogeneity in reporting practice and implementation details, often omitting key data relevant to our framework. Zaccaro and colleagues propose a checklist of data points to include in breath studies [52], including factors such as breath pace, inspiration/expiration ratio, pauses/holds duration, and others. Studies should also report on implementation components, such as human-guided training, number of sessions, practice compliance, session durations, group-versus-individual practice, etc. In the comparative studies included in this review, lack of active controls may have biased results with placebo effects. Future research should compare breath practices with other self-care interventions that are framed as helping with stress reduction.
Although we aimed to address breathing practices performed in isolation and accessible to broad populations, results may have differed if we had included those combined with other practices such as yoga, meditation, or biofeedback. For example, HRV is a well-established stress measure [120]. Changes in self-reported stress may differ when participants see HRV responses and modify their breath pace to achieve certain HRV outcomes. More research is needed comparing biofeedback-enabled breath practices with breath practices alone to help disentangle the cause-and-effect relationships between breath practices, HRV, and stress. Physiological outcomes were also not considered in our analysis. While these outcomes are helpful for understanding breathing practices’ mechanisms of action, our goal was to develop a framework for practical application, regardless of the mechanism of action.
The framework presented here is based on associations between breath practice components and effectiveness; it does not necessarily represent causal relationships. A meta-analysis that pools all data into one larger analysis was not conducted due to heterogeneity in populations, breath practice details, outcome metrics, and statistical approaches in included studies. While existing meta-analyses provide valuable insights [24,35,121], we included a broader array of studies to establish a deeper understanding of how various practice and implementation factors are associated with stress reduction.
Although our results suggest that any breath pace besides fast-only can be effective when the framework criteria are applied, there may be finer distinctions among populations and specific outcomes of interest in which different practices help specific populations in different ways. More carefully controlled and methodologically rigorous experiments may reveal whether these more nuanced distinctions exist.
The potential caveats identified in this framework are hypotheses based on data available in outlier interventions; it could be that other factors drove effectiveness in these interventions. Only two databases were searched for this systematic review; however, the large number of identified and included studies lends credence to the conclusions presented. Lastly, caution is warranted in applying these results to individuals with certain clinical conditions, such as epilepsy, heart conditions, or pregnancy, whose reactions to intense practices may vary.
4.7. Implications
We have identified simple yet important criteria for learning and implementing breath practices for effective stress reduction. Our research demonstrates that the important factors to consider when implementing breath practices for stress reduction are avoiding fast-only and <5 min practices and incorporating appropriate training and continued practice. Extensive standing, interruptions, involuntarily restricted diaphragmatic movement, and inadequate training for highly technical practices may render otherwise promising interventions ineffective. Besides these factors, practitioners and program developers can be less concerned with the specific details of a breath practice, such as finer distinctions in pacing, durations beyond 5 min, group or individual practice, and setting. While these still need to be considered in implementing any breath practice, our findings indicate that over-emphasis on these details is unwarranted and a misdirected placement of focus.
This knowledge can and should provide a sense of freedom for individual practitioners and program developers alike in tailoring programs to meet their needs for stress reduction effectiveness. While many people may benefit from a simple breath practice, some may be reluctant to start for fear of doing the “wrong method.” Our results not only support the broad-ranging benefits of a variety of simple breathing techniques but also provide comfort in the knowledge that, as long as this simple framework is followed, other details are less important for maximizing stress reduction benefits of breathing practices. Specific practices to consider include extended exhale breathing, box breathing, slow breathing with pauses/holds, slow diaphragmatic breathing, combined fast + slower breathing practices, and ANB/UNB. Both initial training sessions and ongoing practice sessions can be as short as 5 min. Breath practice programs should incorporate initial human-guided training and be clear about the specific long-term practice criteria for success.
Future breath practice studies should incorporate rigorous data collection and report breath practice implementation details to allow for analysis of finer practice distinctions and this framework’s validity. Real-time guidance in these studies should be carried out by individuals blinded to experimental conditions, and practices should continue throughout the measurement period. Accountability for, motivation for, and assessment of practice compliance should be addressed, especially for home practices and longer-term follow-up periods. Until more such research is conducted, following the framework results from this analysis will improve stress management effects associated with breathing practices.
4.8. Actionable Takeaways
Following the below framework criteria will increase the likelihood of beneficial stress outcomes associated with breath practices (Table 4).
Table 4.
Actionable takeaways.
| For Practitioners, Program Developers, and Leaders Designing and Implementing Breath Practices | For Researchers Designing and Implementing Breath Practice Studies |
|---|---|
| Pace: ◦Choose any breath pace that incorporates some form of regulated slow breathing, such as: ◾extended exhales; box breathing; slow breathing with pauses/holds; slow diaphragmatic breathing; combined fast+slower breathing; ANB/UNB ◦Avoid practices involving fast breathing alone Training: ◦For at least the 1st session, follow human-voice guidance ◦This can be in-person or via live or recorded video/audio ◦It can be as short as 5 min ◦Get extra training if doing ANB/UNB for the first time Setup: ◦Be in a comfortable seated or lying down position ◦Avoid extensive standing ◦Have access to free diaphragmatic movement ◦Be in a place where you will not be interrupted Practice: ◦Avoid practices shorter than 5 min ◦Do it more than once ◦Keep doing it for continued benefit | Breath practice design: Follow the practice tips in left-hand column Study design: ◦For real-time practice guidance, use instructors or guides who are blinded to experimental condition ◦Create accountability for, motivate, and assess practice compliance, especially for: ◾home practices ◾longer-term follow-up periods ◦Ensure that practices are continued throughout outcome measurement periods |
5. Conclusions
We developed an evidence-based framework for effectively implementing stress reduction breath practices. Despite prior knowledge that breathing practices support stress reduction, how these effects vary across populations, breath practice types, and implementation approaches remained unclear. While commonly emphasized factors such as specific breath pace, population, and group versus individual practice were not found to be associated with effectiveness, five core components were: avoiding fast-only and <5 min practices; and incorporating human-guided training during initial sessions, multiple sessions, and long-term practice. Following this simple, evidence-based framework can help maximize the stress reduction benefits of breathing practices among broad populations.
Acknowledgments
We thank Andrew Galpin, Blerida Banushi, Jill Miller, Katharina Kessel, and Robert Fortune for their insights and contributions during the development of this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13121612/s1, File S1 contains: Table S1: Main data synthesis; Table S2: Framework components for each of the 72 interventions. File S2 provides R-markdown output, showing contingency tables and statistical tests of independence results for all parameters reported in Table 2 in the main text.
Appendix A
Appendix A.1. PubMed and ScienceDirect Search Details
PubMed keywords: anxiety, stress, panic, breath, breathing, breathwork, respiration, respiratory, pranayama, inspiratory, expiratory, and diaphragmatic.
- PubMed MeSH terms [“OR” between all]
- Anxiety/analysis
- Anxiety/etiology
- Anxiety/pathology
- Anxiety/physiology
- Anxiety/physiopathology
- Anxiety/rehabilitation
- Anxiety/therapy
- Stress, Psychological/analysis
- Stress, Psychological/physiology
- Stress, Psychological/physiopathology
- Stress, Psychological/prevention, and control
- Stress, Psychological/psychology
- Stress, Psychological/rehabilitation
- Stress, Psychological/therapy
- panic
- panic/physiology
- panic/prevention and control
- panic/therapy
- panic disorder/etiology
- panic disorder/physiology
- panic disorder/physiopathology
- panic disorder/prevention and control
- panic disorder/therapy
- breathing exercises*
- respiration*
- Diaphragm/therapy
- PubMed Search String:
((((((((((((((“Anxiety/analysis”[Mesh] OR “Anxiety/etiology”[Mesh] OR “Anxiety/pathology”[Mesh] OR “Anxiety/physiology”[Mesh] OR “Anxiety/physiopathology”[Mesh] OR “Anxiety/prevention and control”[Mesh] OR “Anxiety/psychology”[Mesh] OR “Anxiety/rehabilitation”[Mesh] OR “Anxiety/therapy”[Mesh])))) OR (“Stress, Psychological/analysis”[Mesh] OR “Stress, Psychological/education”[Mesh] OR “Stress, Psychological/etiology”[Mesh] OR “Stress, Psychological/pathology”[Mesh] OR “Stress, Psychological/physiology”[Mesh] OR “Stress, Psychological/physiopathology”[Mesh] OR “Stress, Psychological/prevention and control”[Mesh] OR “Stress, Psychological/psychology”[Mesh] OR “Stress, Psychological/rehabilitation”[Mesh] OR “Stress, Psychological/therapy”[Mesh])))) OR (((“panic”[MeSH Terms] OR “panic/physiology”[MeSH Terms] OR “panic/prevention and control”[MeSH Terms] OR “panic/therapy”[MeSH Terms] OR “panic disorder/etiology”[MeSH Terms] OR “panic disorder/physiology”[MeSH Terms] OR “panic disorder/physiopathology”[MeSH Terms] OR “panic disorder/prevention and control”[MeSH Terms] OR “panic disorder/therapy”[MeSH Terms]))))) AND ((((“breathing exercises”[MeSH Terms]) OR “respiration”[MeSH Terms])) OR “Diaphragm/therapy”[Mesh])) AND English[lang])) AND English[lang])) OR ((((((anxiety[Title/Abstract] OR stress[Title/Abstract] OR panic[Title/Abstract])) AND English[lang])) AND (((breath[Title/Abstract] OR breathing[Title/Abstract] OR breathwork[Title/Abstract] OR respiration[Title/Abstract] OR respiratory[Title/Abstract] OR pranayama[Title/Abstract] OR inspiratory[Title/Abstract] OR expiratory[Title/Abstract] OR diaphragmatic[Title/Abstract])) AND English[lang])) AND English[lang])
- PubMed filters:
- English Language
- PubMed study types:
- clinical trial
- clinical study
- controlled clinical trial
- pragmatic clinical trial
- randomized controlled trial
- ScienceDirect study types:
- Research studies
- ScienceDirect title keywords:
(“breath” OR “breathwork” OR “pranayama” OR “respiration” OR “respiratory” OR “diaphragmatic”) AND (“stress” OR “anxiety” OR “panic”).
Appendix A.2. Data Points Abstracted from Articles Accepted for Review
The following data were collected from each article included in the review:
General Study and Intervention Data Abstraction
- Study (i.e., Timestamp, RefID, Author, Title)
- Is the psychological (e.g., survey-based) stress/anxiety measure the primary outcome?
- Journal
- Year
- Study design (e.g., RCT, non-randomized prospective trial, etc.)
- Inclusion criteria
- Exclusion criteria
- Other population descriptions
- Setting
- Geographic location
- Sample size: Intervention (Intervention)—randomized
- Sample size: Intervention (Intervention)—at baseline
- Sample size: Intervention—# completed
- Sample size: control—randomized
- Sample size: control—at baseline
- Sample size: control—# completed
- Sample size: notes
- % Female: overall
- % Female: Intervention
- % Female: control
- Subgroups considered
- Age: Mean—Overall
- Age: Mean—Intervention
- Age: Mean—control
- Race/ethnicity (% each)
- Intervention type
- Intervention other: parameters, components. etc. (What situations is breath protocol being used?)
- Control description
- Measurement tools: stress/anxiety-related
- Measurement tools: other
- Results: stress/anxiety-related
- Results: other metrics
- Subgroup results
- Limitations
- Implications
- Notes
- CLEAR Score of study quality
- Study population category
Framework Data Abstraction
- Significant Benefit for Stress Outcome (yes/no)
- Intervention: breathing type (i.e., slow, fast, combined fast-slow, other)
- Slow breathing type(s) used
- Describe fast breathing practice (if used)
- Intervention breath pattern
- Breaths per minute (bpm) in breath practice
- Note regarding bpm
- Human-guided training (yes/no)
- Type of human-guided training
- Number of human-guided training sessions
- Number of minutes per human-guided training session
- Device-guided breath training/pacing (yes/no)
- Device type
- Number of device-guided training sessions
- Number of minutes per device-guided training session
- Intervention: group or individual
- Number of group sessions
- Number of minutes per group session
- Intervention homework (yes/no)
- Intervention homework description
- Total number of homework breath sessions
- Length of homework breath sessions (minutes)
- Intervention duration (days)
- Multiple (≥1) sessions (yes/no)
- Number of breath practice sessions, total known
- Length of general sessions (minutes)
- Length of group sessions, if done (minutes)
- Long-term practice (yes/no)
- Sessions continued through outcome measurement (yes/no)
- Control includes (e.g., no control/usual care/no intervention)
- At least one framework criterion met (yes/no)
- Framework: effectiveness of breathing practice
- Which criteria were included
- How many criteria were included
- Randomized controlled trial (yes/no)
- Primary outcome: stress/anxiety (yes/no)
- How many times were stress/anxiety outcomes measured
- Participants: # total randomized
Appendix A.3. Modified CLEAR-NPT Quality Assessment Tool [51,62]
CLEAR-NPT uses a checklist of 10 specific quality assessment criteria regarding blinding, group allocation, care providers’ experience/skill, participant adherence, and reporting details to evaluate reports of nonpharmacological treatments. The modified form of the checklist used in this review includes an additional question regarding the comparability of treatment and control groups. Due to the difficulty of blinding in behavioral interventions, the modified form also provides alternative quality assessment questions when blinding is not possible for participants or clinicians. Quality score is calculated by dividing the number of met criteria by the number of applied criteria for a final quality index ranging from 0.0 to 1.0. Because some criteria may not apply to all studies, this approach allows for a more valid comparison across studies.
Table A1.
Modified CLEAR-NPT quality assessment tool questions.
| | Criterion | Yes | No | Unclear | | | ------------ | -------------------------------------------------------------------------------------------------------------------------------------------------------- | -- | ------- | | | 1 | Was the generation of allocation sequences (group assignment procedure) adequate? | | | | | 2 | Was the treatment allocation concealed? | | | | | 3 | Were details of the intervention administered to each group stated or made available? | | | | | 4 | Were care providers’ experience or skills in each arm (group) appropriate? | | | | | 5 | Was participant adherence or compliance assessed quantitatively? | | | | | 6 | Were participants adequately blinded? If NO, please go to 6.1.1 and 6.1.2. If YES, please go to 7 | | | | | 6.1.1 | Were other treatments and care (i.e., co-interventions) the same in each randomized group? | | | | | 6.1.2 | Were withdrawals and lost-to-follow-up the same in each randomized group? | | | | | 7 | Were care providers for the participants adequately blinded? If NO, please go to 7.1.1 and 7.1.2. If YES, please go to 8. | | | | | 7.1.1 | Were all other treatments and care (co-interventions) the same in each randomized group? | | | | | 7.1.2 | Were withdrawals and lost-to-follow-up the same in each randomized group? | | | | | 8 | Were outcome assessors adequately blinded to assess the primary outcomes? | | | | | 8.1 | If outcome assessors were not adequately blinded, were specific methods used to avoid ascertainment bias (systematic differences in outcome assessment)? | | | | | 9 | Was the follow-up schedule the same in each group? (parallel design) | | | | | 10 | Were the treatment and control group comparable at entry? (Any significant differences at baseline?) | | | | | 11 | Were the main outcomes analyzed according to the intention-to-treat principle? | | | | | Total | Total Scores (number of Yes) | | | |
Appendix A.4. National Heart Lung Blood Institute-NHLBI/National Institutes of Health-NIH Quality Assessment Tool [63] for Pre-Post Studies with No Control Group
This tool provides a qualitative evaluation of studies’ internal validity by using questions about the clarity of study objectives, adequacy of the study population and sample size, delivery and description of the intervention, appropriateness of statistical methods, and other metrics. Each study’s quality score is based on the total number of “yes” answers divided by total number of questions answered, and that score is used to assign each article with a quality assessment ranking of good, fair, or poor using the same scoring as that used for CLEAR-NPT: <0.6, poor quality; 0.6–0.8 fair; and >0.80 good.
Guidance for using NHLBI-NIH Quality Assessment Tool:
“The questions in the quality assessment tool were designed to help reviewers focus on the key concepts for evaluating the internal validity of a study. They are not intended to create a list from which to add up items to judge a study’s quality.
Internal validity is the extent to which the outcome results reported in the study can truly be attributed to the intervention or exposure being evaluated and not to biases, measurement errors, or other confounding factors that may result from flaws in the design or conduct of the study. In other words, what is the ability of the study to draw associative conclusions about the effects of the interventions or exposures on outcomes?
Critical appraisal of a study involves considering the risk of potential for selection bias, information bias, measurement bias, or confounding (the mixture of exposures that one cannot tease out from each other). Examples of confounding include co-interventions, differences at baseline in patient characteristics, and other issues throughout the questions above. A high risk of bias translates to a rating of poor quality; a low risk of bias translates to a rating of good quality. Again, the greater the risk of bias, the lower the quality rating of the study.
In addition, the more attention in the study design to issues that can help determine if there is a causal relationship between the exposure and outcome, the higher quality of the study. These issues include exposures occurring prior to outcomes, evaluation of a dose-response gradient, accuracy of measurement of both exposure and outcome and sufficient timeframe to see an effect.
Generally, when reviewers evaluate a study, they will not see a “fatal flaw” but instead will find some risk of bias. By focusing on the concepts underlying the questions in the quality assessment tool, reviewers should ask themselves about the potential for bias in the study they are critically appraising. For any box checked “no” reviewers should ask, “What is the potential risk of bias resulting from this flaw in study design or execution?” That is, does this factor lead to doubt about the results reported in the study or doubt about the ability of the study to accurately assess an association between the intervention or exposure and the outcome?
The best approach is to think about the questions in the assessment tool and how each one reveals something about the potential for bias in a study. Specific rules are not useful, as each study has specific nuances. In addition, being familiar with the key concepts will help reviewers be more comfortable with critical appraisal. Examples of studies rated good, fair, and poor are useful, but each study must be assessed on its own.”.
Questions:
- Was the study question or objective clearly stated?
- Were eligibility/selection criteria for the study population prespecified and clearly described?
- Were the participants in the study representative of those who would be eligible for the test/service/intervention in the general or clinical population of interest?
- Were all eligible participants that met the prespecified entry criteria enrolled?
- Was the sample size sufficiently large to provide confidence in the findings?
- Was the test/service/intervention clearly described and delivered consistently across the study population?
- Were the outcome measures prespecified, clearly defined, valid, reliable, and assessed consistently across all study participants?
- Were the people assessing the outcomes blinded to the participants’ exposures/interventions?
- Was the loss to follow-up after baseline 20% or less? Were those lost to follow-up accounted for in the analysis?
- Did the statistical methods examine changes in outcome measures from before to after the intervention? Were statistical tests carried out that provided _p_-values for the pre-to-post changes?
- Were outcome measures of interest taken multiple times before the intervention and multiple times after the intervention (i.e., did they use an interrupted time-series design)?
- If the intervention was conducted at a group level (e.g., a whole hospital, a community, etc.), did the statistical analysis take into account the use of individual-level data to determine effects at the group level?
Table A2.
Stress/anxiety metrics and outcomes of included studies (N = 58) by population and use of stress/anxiety as the primary or secondary outcome. Bolded phrases in “Outcome” column reflect key stress/anxiety intervention outcomes.
| Author, Year, Setting | Stress/Anxiety Metric | Outcomes ** | Quality Score, Tool † [62,63] |
|---|---|---|---|
| A. Youth | |||
| Youth: Stress/anxiety as primary outcome | |||
| * Sellakumar, 2015 [20] India, secondary school (grades 6–12) | STAI-state, trait | After Ix: Ix but not C had significant decreases in state and trait anxiety (p < 0.001 for both) These reductions were significantly greater in Ix than in C (p < 0.001 for state and trait) | 0.44(CLEAR)Poor |
| * Khng, 2017 [66] Singapore, 4 elementary schools | STAI-C-state | When doing test after and w/Ix: Ix had significantly greater anxiety reduction vs. C (p = 0.02) Ix had significantly larger increases in math scores (p = 0.04) | 0.57(CLEAR)Poor |
| * Bargale, 2021 [67]India, hospital pediatric dentistry dept. | Animated emoji scale | From before to after anesthetic procedure, significant anxiety decrease in both groups (p < 0.001) w/no significant differences between groups (_p_ > 0.001) | 0.86(CLEAR)Good |
| Youth: Stress/anxiety as secondary outcome | |||
| * Hakked, 2017 [68] India, swim academy | SAS-2 | From before to after Ix: Ix improved significantly more than C in: max voluntary ventilation (p = 0.02); forced vital capacity (p = 0.026); swim strokes per breath (p = 0.002) Ix but not C significantly improved anxiety (p = 0.031), concentration (p = 0.022), somatic complaints (p = 0.037), inspiratory reserve volume (p < 0.05), and swim strokes per breath (p ≤ 0.001) | 0.69(CLEAR)Fair |
| B. Healthy Adults | |||
| Healthy Adults: Stress/anxiety as primary outcome | |||
| * Gupta, 2010 [69]India, yoga training camp | Sinha Anxiety Scale | From before to after Ix, significant decrease in mean anxiety and BDI (p < 0.01) ‡‡ | 0.25(NHLBI)Poor |
| * Bhimani, 2011 [31] India, medical college | Authors’ 20-item stress questionnaire | From before to after Ix: Total stress score significantly decreased (p = 0.0001) Low- and high-frequency HRV showed decreased sympathetic and increased parasympathetic influences and improved sympathovagal balance (p = 0.0001 all) | 0.27(NHLBI)Poor |
| * Sharma, 2013 [70]India, yoga research center and school | PSS | From baseline to 12 wks: Significant PSS decrease in Ix-1 and Ix-2 (p = 0.000 both) Significant improvement in cardiovascular measures for Ix-2 (p < 0.05) but not Ix-1 (_p_ > 0.09) | 0.66(CLEAR)Fair |
| * Sundram 2014 [71] Malaysia, automotive assembly plant | DASS-21 stress subscale | 9-mo DASS-21 was significant: Reduced from baseline in Ix and C for all subscales (p < 0.001), and this difference was significantly greater in Ix than C for stress subscale (p < 0.001) Differences between Ix and C (p < 0.001) | 0.62(NHLBI)Fair |
| * Schmalzl, 2018 [72] US, university town | PSS | Following the 8 wk program, significant: PSS decrease in Ix and C (p < 0.01) Salivary cortisol decrease in Ix and C (_p_ < 0.01) Improved visual discrimination task in Ix and C (_p_ = 0.017) Improved overall perceptual sensitivity in C (_p_ < 0.01) but not Ix (_p_ > 0.05) | 0.75(CLEAR)Fair |
| * Okado, 2020 [73]US, university setting | PSS, GAD-7 | From baseline to 2 wks: PSS significantly decreased for Ix-1 (p < 0.01), Ix-2 (_p_ < 0.05), and Ix-3 (_p_ < 005), but not C (_p_ > 0.05) GAD-7 significantly decreased for Ix-1 (p < 0.05) PHQ-15 somatization significantly decreased in Ix-2 (p < 0.05) and Ix-3 (p < 0.05) PHQ-9-M depression significantly decreased for Ix-1 (p < 0.01) and Ix-2 (p < 0.05) | 0.31(CLEAR)Poor |
| * Magnon, 2021 [74]France, setting unclear | SAI | SAI significantly lower post-Ix among entire group (p < 0.001), young adults (p = 0.003), and older adults (p = 0.019) Significant increase in HF power after Ix (p < 0.001), which was greater in older adults than younger (p = 0.042) | 0.67(NHLBI)Fair |
| * Balban, 2023 [75]US, remote setting | STAI | Ix-1, Ix-2, and Ix-3 had significant improvement in mood and lower respiratory rate compared w/C (p < 0.05 for all) **Averaged over 28 days, all groups significantly reduced state anxiety pre- to post-practice** (_p_ < 0.0001 all) No significant change in trait anxiety in any groups, nor differences between groups (_p_ > 0.05) | 0.53(CLEAR)Poor |
| Healthy Adults: Stress/anxiety as secondary outcome | |||
| * Busch, 2012 [76]Germany, university setting | POMStension-anxiety | Following Ix: Tension-anxiety significantly improved in Ix-1 and Ix-2 (p < 0.0001) Ix-1 and Ix-2 significantly improved in depression, anger (_p_ < 0.01 all) and total mood disturbances (_p_ < 0.01 Ix, _p_ < 0.05 Ix-2), and not on vigor, fatigue, or confusion scales (_p_ > 0.05 all) No significantly different between Ix-1 and 2 on any POMS measures (p = 0.55) Ix-1 showed significant increases in pain thresholds (p < 0.001) and reductions in sympathetic activity (p = 0.002) | 0.73(NHBLI)Fair |
| Lin, 2014 [45]Taiwan, university setting | VAS-anxiety | During Ix:Significant differences between all Ix’s for SDNN (p < 0.001), LF (_p_ < 0.001), and LF/HF ratio (_p_ < 0.001), but not HF (_p_ = 0.06) Using retrospective ratings taken after all Ix’s: Significantly greater relaxation for all compared to baseline (_p_ < 0.05 all) **_No significant differences in anxiety_** between any Ix and baseline (_p_ > 0.05 all) | 0.58(NHLBI)Poor |
| * Hunt, 2018 [21]US, large private university | 10-point Likert scale of subjective stress vs. relaxation | Compared w/C, Ix resulted in significantly higher HRV (p < 0.001), lower respiration rate (p < 0.001), higher tidal volume (p = 0.001), and lower HR (p < 0.001) Stress was significantly lower after Ix and C (p < 0.001 both) than after paced breathing alone No significant stress differences after Ix vs. C (p = 0.14) No significant differences between Ix and C in any outcomes during cognitive stressor challenge (_p_-value not reported) | 0.56(CLEAR)Poor |
| * Naik, 2018 [77] India, medical school | PSS | After 12 wks, Ix but not C had significant: Improvements in HR, SBP, DBP (p < 0.05), not BMI and WHR (_p_ > 0.05) Decrease in PSS (p < 0.0001) Compared to C, Ix had greater PSS decrease (p < 0.05) | 0.62(CLEAR)Fair |
| * Conlon, 2022 [78]UK, University of Bath | IAMS, 7-point Likert scale of perceived stress | No significant Ix effects on shooting performance (p > 0.05) Post-Ix: State anxiety significantly decreased in Ix-1 (p < 0.001) **and Ix-2** (_p_ = 0.018) but not C (_p_ > 0.05) Perceived stress significantly decreased in Ix-1 (p < 0.001) **and Ix-2** (_p_ = 0.016) but not C (_p_ > 0.05) | 100(CLEAR)Good |
| * Schlatter, 2022 [79]France, university medical school | VAS-stress | Overall performance was significantly greater in Ix-1 (p = 0.013) and Ix-2 Ix-2 (p = 0.009) vs. C Clinical performance was significantly greater in Ix-1 (p = 0.009) but not Ix-2 (p = 0.073) vs. C No group differences in non-technical skills (p = 0.285) After the Ix, but before the simulation: VAS-stress significantly lower in Ix-1 (p = 0.029) and Ix-2 (p = 0.035) vs. C, but no differences between Ix’s (p = 0.897) Thayer relaxation significantly lower in Ix-1 (p = 0.0.01) and Ix-2 (p = 0.0.035) vs. C, but no differences between Ix’s (p = 0.709) No differences in Thayer tension between Ix-1 (p = 0.275) and Ix-2 (p = 0.213) vs. C or between Ix’s (p = 0.875) | 0.90(CLEAR)Good |
| C. High-Anxiety Populations | |||
| High-Anxiety Populations: Stress/anxiety primary outcome | |||
| * Clark, 1990 [80] US, inpatient alcohol rehabilitation facility | STAI-state | STAI-state anxiety scores and self-reported tension significantly decreased after each session for Ix and C (p < 0.0001 both metrics, groups) Ix had greater improvements than C in STAI-state (p < 0.04) and tension (p < 0.0002) Ix had significantly lower STAI-state scores than C following both sessions (p < 0.01 both) | 0.50(CLEAR)Poor |
| * Chen, 2017 [81]Taiwan, medical center | BAI | Ix significantly reduced BAI anxiety between wks 0–4 (p = 0.001), 0-8 (p < 0.001), and 4–8 (_p_ < 0.001); C did not (_p_ > 0.05) BAI was significantly lower for Ix than C at wk 8 (p = 0.033) Ix but not C improved significantly from wks 0-8 in skin conductivity (p = 0.48), peripheral temperature (p = 0.007), HR (p < 0.0001), and breathing rate (p < 0.0001) | 0.69(CLEAR)Fair |
| * Serafim, 2018 [82] Brazil, hospital | HAM-A, BAI | After the Ix and at 10-wk follow-up: HAM-A and BAI were significantly lower (p < 0.001 for both) Depression and mania ratings were significantly lower (p < 0.001 and p = 0.027, respectively) | 0.50(NHBLI)Poor |
| High-Anxiety Populations: Stress/anxiety as secondary outcome | |||
| * Clark, 1985 [83] UK, setting unclear | 100-pt Likert scale, MMFQ | Compared w/before Ix: Panic attack frequency significantly reduced during Ix (p < 0.01) and 2–13 wks after (_p_ < 0.05) **Anxiety levels significantly lower** (_p_ < 0.01) during 15 min exposure to anxiety-provoking situation **Anxiety/depression** (_p_ < 0.001) and global distress (_p_ < 0.05) significantly lower No significant changes in BDI depression (_p_ > 0.05) | 0.73(NHBLI)Fair |
| D. Clinical Populations (Acute) | |||
| Clinical Populations (Acute): Stress/anxiety as primary outcome | |||
| Biggs 2003 [40] US, private dental practice | DAR-S | No significant differences between any groups overall in dental-related anxiety (p = 0.67), post-Ix anxiety (p = 0.46), or anxiety reduction, including C C participants who used own anxiety reduction technique reported significantly less anxiety (p = 0.04) | 0.38(CLEAR)Poor |
| * Hayama, 2012 [84]Japan, hospital inpatient | Short-form POMS (Japanese version) | Median post-chemo POMS tension-anxiety significantly lower among Ix vs. C (p = 0.01) Both Ix and C showed significant reductions in pre-post chemo POMS tension-anxiety (both p < 0.01) | 0.69(CLEAR)Fair |
| * Valenza, 2014 [85]Spain, hospital | HADS-anxiety | Following Ix, all variables were better and improved in Ix, w/significantly improved HADS anxiety, dyspnea, and mobility (p < 0.001 for all) ‡ | 0.63(CLEAR)Fair |
| * Bidgoli, 2016 [86]Iran, hospital | SAI | Compared w/before Ix: Ix but not C had significantly reduced anxiety at 30 and 60 min after Ix (p = 0.0001 both) Anxiety decreases significantly greater in Ix than C (p < 0.05) | 0.53(CLEAR)Poor |
| Boaviagem, 2017 [41]Brazil, maternity hospital | STAI (unclear whether state or trait) | No differences Ix vs. C in mean STAI (p = 0.58 per-protocol, p = 0.91 ITT) No differences in maternal pain/satisfaction or fatigue; delivery mode; Apgar scores (p > 0.05 all) | 0.75(CLEAR)Fair |
| * Cicek, 2017 [87]Turkey, hospital delivery room | STAI-state | Mean STAI-state significantly lower (p < 0.001) **in Ix vs. C for late active labor but not early or latent** (_p_ > 0.05) All labor phases (early latent, late latent, active) except transitional were shorter (p < 0.05) in Ix vs. C | 0.69(CLEAR)Fair |
| * Chandrababu, 2019 [88] India, hospital | STAI-state | From before surgery to 5th post-op day: STAI-state significantly reduced in Ix (p = 0.001) but not C This reduction was significantly greater in Ix than C (p = 0.001) Ix had significantly less pain than C on 3rd post-op day (p < 0.05) No between-group differences in pain on 4th or 5th days (_p_ > 0.05) | 0.73(NHLBI)Fair |
| Ratcliff, 2019 [47]US, university medical center | VAS-anxiety, STAI-state | Reduction in VAS anxiety during biopsy was: Significantly greater in Ix-1 than Ix-2 (p < 0.01) and C (p < 0.0001) Not significant differences between Ix-2 and C (p = 0.65) Ix-1 had significant increase in beta current source density (CSD) in insula (p = 0.006) and ACC (p = 0.019) compared to C | 0.64(CLEAR)Fair |
| * Grinberg, 2020 [89]US, medical center | STAI-state | Compared to C, Ix group had significant: Lower post-procedural STAI-state (p = 0.039) Larger STAI-state decrease pre- to post-procedure (p < 0.000) Lower HR post-procedure (_p_ = 0.048) Larger HR decrease pre- to post-procedure (_p_ = 0.01) No significant BP changes or differences (_p_ > 0.1 all) | 0.50(NHLBI)Poor |
| * Abo El Ata AB, 2021 [90]Egypt, hospital | Burn-specific pain anxiety scale | Significantly reduced burn-specific pain anxiety: Pre-to-post breathing exercises (p = 0.000) Wk 1 (pre-Ix) to wks 2 and 3 (during Ix; p = 0.000) From pre- to post-breathing exercises, significantly reduced: Pain (p = 0.000) SBP (p = 0.000), DBP (p = 0.000), respiratory rate (p = 0.000), HR (p = 0.000) From pre-post significantly increased relaxed behavior (p < 0.002) | 0.64(NHLBI)Fair |
| * Hosseinzadeh-Karimkoshteh, 2021 [91]Iran, medical center | Burn patient anxiety inventory | On each of the 4 days, Ix had significantly lower pain anxiety than C (p < 0.001 all) | 0.78(CLEAR)Fair |
| * Zahn, 2021 [92]Switzerland, university hospital | STAI, APAIS,VAS-stress | Compared w/C, Ix intraoperative anxiety was: Significantly lower for all measures (STAI-state, p = 0.0001; APAIS, p = 0.0003; VAS, p = 0.0007) Significantly lower vs. pre-op for STAI-state (p = 0.007) and APAIS (p = 0.009), but not VAS (p = 0.79) | 0.64(CLEAR)Fair |
| * Moghadam, 2022 [93]Iran, psychiatric hospital | BAI | Mean anxiety was significantly lower post-Ix: Compared w/pre-Ix in both Ix’s (p < 0.001) but not C (p = 0.10) In both Ix groups vs. C (p < 0.001) | 0.56(CLEAR)Poor |
| * Aktas, 2023 [39]Turkey, Ankara Hospital, | STAI | From pre-op to post-Ix: STAI-state significantly decreased in Ix-1 (p = 0.007) but not Ix-2 or C QoL significantly increased in Ix-2 (p = 0.043 both) and C (p = 0.003) but not Ix-1 Post-Ix, significantly different in STAI-state across groups (p < 0.05) | 0.37(CLEAR)Poor |
| Clinical Populations (Acute): Stress/anxiety as secondary outcome | |||
| * Dhruva, 2012 [94] US, university medical center | HADS-anxiety | Ix was feasible (close to 100% compliance) Sleep disturbance (p = 0.04) and anxiety (p = 0.04) improved w/every h of pranayama practiced during Ix period From before-to-after 1st chemo cycle, no significant differences Ix vs. C in stress or other outcomes (p > 0.05 all) | 0.63(CLEAR)Fair |
| * Park, 2013 [95]South Korea, medical center | VAS-anxiety | Compared w/C and over the 3 days, Ix had significant: Lower pain scores (p = 0.01) and reduction in pain scores (p = 0.001) Lower anxiety (p = 0.01) and reduction in anxiety (p = 0.001) Ix had significantly reduced anxiety over the 3 days (p = 0.02) | 0.67(NHLBI)Fair |
| * Eldin, 2015 [96]Libya, hospital | STAI-state | Post-Ix, Ix but not C had significant: STAI decrease all 3 days (p = 0.003 day 1, p < 0.001 days 2 and 3), and decrease was significantly greater than in C (p < 0.001) Pain and pain behavior decreases (p < 0.001 both) Improvement in respiration, HR, and BP (p < 0.001 all) | 0.55(NHLBI)Poor |
| * Iyer, 2020 [97]India, medical center | VAS-anxiety, PASS | Compared w/pre-Ix, significant decreases found after 1 and 7 days in (p = 0.00 all): Pain Anxiety Anticipatory anxiety | 0.73(NHLBI)Fair |
| * Ursavas, 2020 [98]Turkey, hospital | HADS-anxiety | At 1,2,4,8,12, and 24 h post-op: Pain: No significant between-group differences at any time point (p > 0.05 all) Compared to C, anxiety significantly lower in Ix (p = 0.033) Depression: No significant differences (p = 0.081) | 0.68(CLEAR)Fair |
| * Lu, 2022 [99]China, hospital | HADS | Compared w/C, Ix had significant differences in: Dyspnea at T1 (p = 0.037), T3 (p = 0.003) HADS anxiety reductions at T1 day before surgery (p = 0.046), T2, day after surgery (p = 0.02), T3 at discharge (p < 0.001) Post-op complications (_p_ = 0.045) No between-group differences in 6 min walk test times at any time point (_p_ > 0.05) | 0.73(CLEAR)Fair |
| E. Clinical Populations (Chronic) | |||
| Clinical Populations (Chronic): Stress/anxiety as primary outcome | |||
| * Marshall, 2014 [100] US, setting unclear | BAI | BAI was significantly lower after 4 wks (p = 0.0069) Among those w/o aphasia, COWAT-measured brain function, and verbal abilities significantly improved after 10 wks (p = 0.0001) Among those w/aphasia, some speech-language measures significantly improved after 4 wks of guided practice (p < 0.05) | 0.55(NHLBI)Fair |
| Clinical Populations (Chronic): Stress/anxiety as secondary outcome | |||
| * Han, 1996 [22] Belgium, setting unclear | STAI-state and trait | From before to after Ix:HVS complaints significantly reduced (p < 0.001), based on Nijmegen Questionnaire Inspiratory and expiratory time and tidal volume increased significantly (_p_ < 0.001), but not end-tidal CO2 (_p_ > 0.05) State (p < 0.001) **but not trait** (_p_ > 0.05) anxiety significantly reduced | 0.73(NHLBI)Fair |
| * Thomas, 2009 [101] UK, primary care general practices | HADS-anxiety | At 6 mo following Ix, Ix had significantly greater improvements than C in AQLQ (p = 0.01), HADS anxiety (p = 0.02), and depression (p = 0.03), and hyperventilation symptoms (p = 0.005) No significant differences between groups in AQLQ, HADS anxiety or depression at 1 mo (p > 0.05 all) Breathing training did not significantly change airway physiology, inflammation, or hyper-responsiveness (p > 0.05 all) | 0.73(CLEAR)Fair |
| Jefferson, 2010 [43]US, setting unclear | PSS, STAI | SBP and DBP were decreased for Ix at 1-wk follow-up (p = 0.0001 and 0.02, respectively) but not 6 wks, and not for C (p > 0.05) No significant differences after Ix in PSS or STAI-state anxiety for Ix or C (p > 0.05 all) STAI-trait anxiety significantly decreased for Ix (p = 0.01) but not C (p = 0.09) at 1-wk post-Ix, but not at 6 wks (p > 0.05 both) | 0.25(CLEAR)Poor |
| * Sureka, 2014 [102] India, prison hospital | PGWBS-anxiety | After 6 wks, Ix vs. C had significantly greater improvements in: Global functioning, depression, positive well-being, psychological general well-being, and general health (p < 0.01 for all) PGWBS-anxiety (p < 0.01) | 0.80(CLEAR)Fair |
| * Sureka, 2015 [103]India, prison hospital | PGWBS-anxiety | After 6 wks, Ix vs. C significantly improved in: GAF (p = 0.0002) Anxiety (p = 0.011) Positive Well-Being (p = 0.02) General Health (p = 0.02) Total PGWB (p = 0.05) | 0.73(CLEAR)Fair |
| Thomas, 2017 [50] UK, general practices | HADS-anxiety | 12 mo after Ix: AQLQ significantly better in Ix-1 (p < 0.001) and Ix-2 (_p_ < 0.05) vs. C HADS-depression significantly lower in Ix-2 vs. C (_p_ = 0.03) No significant changes in lung function, asthma symptom control, or asthma exacerbation rate vs. C (_p_ > 0.05 all) No significant differences in HADS-anxiety pre-post Ix in all groups or between groups (p > 0.05 all) | 0.80(CLEAR)Fair |
| * Fiskin, 2018 [104]Turkey, medical center | DASS | After 30 days, Ix had: PAI increase (p = 0.000), greater vs. C (p = 0.000) Stress/anxiety decrease (p = 0.000), greater vs. C (p = 0.000) Depression decrease (p = 0.001), greater vs. C (p = 0.003) | 0.50(CLEAR)Poor |
| * Watson, 2022 [105]New Zealand hospital | 5-point Likert Scale | Compared w/Ix-2, Ix-1 significant initial increase in: QTc interval (p < 0.0002) Anxiety (p < 0.001) | 0.75(CLEAR)Fair |
| F. Simulated Stress | |||
| Simulated Stress Populations: Stress/anxiety as primary outcome | |||
| Kamath, 2017 [44] India, medical school | VAMS-anxiety | Ix showed trend (p > 0.05) toward lower VAMS anxiety prior to, during, and following public speaking task | 0.88(CLEAR)Good |
| Simulated Stress Populations: Stress/anxiety as secondary outcome | |||
| Holmes, 1978 [42] US, university setting | Author-designed 18-item anxiety checklist | After threat or stimulation: HR was significantly higher for threat vs. stimulation (p < 0.001), there were no significant differences between groups in stimulation condition, and it was significantly lower for C than Ix-1 or Ix-2 in threat condition (_p_ < 0.05) Skin resistance did not differ between groups or threat/stimulation conditions (_p_ > 0.1) Significantly less anxiety reported in C vs. Ix-1 and Ix-2 (p < 0.05) No significant differences in anxiety between Ix-1 and Ix-2 (_p_-value not reported) | 0.33(NHLBI)Poor |
| * McCaul, 1979 [106] US, university setting | Author-designed 18-item anxiety checklist | Following threat/stimulation: Ix-1 anxiety significantly lower than in combined Ix-2 and Ix-3 (p = 0.03) Self-reported ability to relax while awaiting shock significantly lower in expectation vs. no-expectation groups (p = 0.02) Ix-1 vs. Ix-2 and Ix-3 combined had significantly lower skin resistance and pulse during threat anticipation (p = 0.05, p = 0.02, respectively) and threat (p = 0.02 both), but not HR (_p_-value not reported) | 0.50(CLEAR)Poor |
| Sakakibara, 1996 [48] Japan, university setting | STAI-state | Following threat anticipation: High frequency HRV during threat stayed the same for Ix-1 and significantly decreased (worsened) for Ix-2 and C (p < 0.05 for both); low-frequency HRV changes did not differ between groups (_p_-value not reported) **_STAI-state anxiety worsened in all groups_**, w/no differences in changes between groups (_p_ > 0.05) | 0.50(CLEAR)Poor |
| * Telles, 2019 [49]India, residential yoga center | STAI-state | From pre- to post- each Ix: Shape and size discrimination task errors significantly reduced after Ix-1 (p < 0.001) STAI significantly reduced after Ix-2 (p < 0.05) and C (p < 0.001) but not Ix-1 | 0.85(CLEAR)Good |
| Meier, 2020 [46]Canada, setting unclear | VAS-stress | In response to Ix, no significant differences between groups for cortisol (p = 0.847), alpha amylase (p = 0.735), or stress (p = 0.986)In response to stressor: Ix-2 had significantly lower cortisol levels vs. C (p = 0.045), but no significant differences for Ix-1 vs. Ix-2 (p = 0.052) or C (p = 0.955) No significant differences between groups for alpha amylase (p = 0.38) or stress (p = 0.818) | 0.81(CLEAR)Good |
| * Sharma, 2022 [107]India, university setting | STAI-state | From pre- to post-Ix: Significant increase in attention task scores in Ix-4 (p < 0.05) **STAI-state scores decreased significantly in Ix-1** (_p_ = 0.001), **Ix-2** (_p_ = 0.008), **Ix-3** (_p_ = 0.002), **Ix-4** (_p_ = 0.042) but not Ix-5 and Ix-6 (_p_ > 0.05) Ix-1 had significantly lower STAI-state scores post-Ix vs. Ix-5 (p < 0.05) | 100(CLEAR)Good |
Author Contributions
Conceptualization, T.G.K.B.; methodology, T.G.K.B. and M.R.; data review and acquisition, T.G.K.B., G.D.-P., M.R., N.A., M.L., R.B., K.C. and P.S.; analysis, T.G.K.B., G.D.-P. and M.R.; writing—original draft preparation, T.G.K.B., G.D.-P., M.R., N.A. and P.S.; writing—review and editing, T.G.K.B., G.D.-P. and M.R.; supervision, T.G.K.B.; project administration, T.G.K.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data synthesis and output available in Supplementary Materials. Other data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Santomauro D.F., Herrera A.M.M., Shadid J., Zheng P., Ashbaugh C., Pigott D.M., Abbafati C., Adolph C., Amlag J.O., Aravkin A.Y., et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuPont R.L., Rice D.P., Miller L.S., Shiraki S.S., Rowland C.R., Harwood H.J. Economic Costs of Anxiety Disorders. Anxiety. 1996;2:167–172. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Kalia M. Assessing the Economic Impact of Stress—The Modern Day Hidden Epidemic. Metabolism. 2002;51:49–53. doi: 10.1053/meta.2002.33193. [DOI] [PubMed] [Google Scholar]
- 5.Niles A.N., O’Donovan A. Comparing Anxiety and Depression to Obesity and Smoking as Predictors of Major Medical Illnesses and Somatic Symptoms. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2019;38:172–181. doi: 10.1037/hea0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang H.-J., Bae K.-Y., Kim S.-W., Shin H.-Y., Shin I.-S., Yoon J.-S., Kim J.-M. Impact of Anxiety and Depression on Physical Health Condition and Disability in an Elderly Korean Population. Psychiatry Investig. 2017;14:240–248. doi: 10.4306/pi.2017.14.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas B.S., Franks P., Ingram D.D. Are Symptoms of Anxiety and Depression Risk Factors for Hypertension? Longitudinal Evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch. Fam. Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Eaker E.D., Sullivan L.M., Kelly-Hayes M., D’Agostino R.B., Benjamin E.J. Tension and Anxiety and the Prediction of the 10-Year Incidence of Coronary Heart Disease, Atrial Fibrillation, and Total Mortality: The Framingham Offspring Study. Psychosom. Med. 2005;67:692–696. doi: 10.1097/01.psy.0000174050.87193.96. [DOI] [PubMed] [Google Scholar]
- 9.Reiche E.M.V., Nunes S.O.V., Morimoto H.K. Stress, Depression, the Immune System, and Cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 10.Rozanski A., Blumenthal J.A., Kaplan J. Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.CIR.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 11.Schneiderman N., Ironson G., Siegel S.D. STRESS AND HEALTH: Psychological, Behavioral, and Biological Determinants. Annu. Rev. Clin. Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The Impact of Stress on Body Function: A Review. EXCLI J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado A., Herrera A.J., de Pablos R.M., Espinosa-Oliva A.M., Sarmiento M., Ayala A., Venero J.L., Santiago M., Villarán R.F., Delgado-Cortés M.J., et al. Chronic Stress as a Risk Factor for Alzheimer’s Disease. Rev. Neurosci. 2014;25:785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- 14.Vyas S., Rodrigues A.J., Silva J.M., Tronche F., Almeida O.F.X., Sousa N., Sotiropoulos I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016;2016:6391686. doi: 10.1155/2016/6391686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen B.S. Central Effects of Stress Hormones in Health and Disease: Understanding the Protective and Damaging Effects of Stress and Stress Mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter J.K., Andrews L.A., Witcraft S.M., Powers M.B., Smits J.A.J., Hofmann S.G. Cognitive Behavioral Therapy for Anxiety and Related Disorders: A Meta-Analysis of Randomized Placebo-Controlled Trials. Depress. Anxiety. 2018;35:502–514. doi: 10.1002/da.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandelow B., Michaelis S., Wedekind D. Treatment of Anxiety Disorders. Dialogues Clin. Neurosci. 2017;19:93–107. doi: 10.31887/DCNS.2017.19.2/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta P. Health Impacts of Yoga and Pranayama: A State-of-the-Art Review. Int. J. Prev. Med. 2012;3:444–458. [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer H., Lauche R., Anheyer D., Pilkington K., de Manincor M., Dobos G., Ward L. Yoga for Anxiety: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Depress. Anxiety. 2018;35:830–843. doi: 10.1002/da.22762. [DOI] [PubMed] [Google Scholar]
- 20.Sellakumar G. Effect of Slow-Deep Breathing Exercise to Reduce Anxiety among Adolescent School Students in a Selected Higher Secondary School in Coimbatore, India. J. Psychol. Educ. Res. (JPER) 2015;23:54–72. [Google Scholar]
- 21.Hunt M.G., Rushton J., Shenberger E., Murayama S. Positive Effects of Diaphragmatic Breathing on Physiological Stress Reactivity in Varsity Athletes. J. Clin. Sport Psychol. 2018;12:27–38. doi: 10.1123/jcsp.2016-0041. [DOI] [Google Scholar]
- 22.Han J.N., Stegen K., De Valck C., Clément J., Van de Woestijne K.P. Influence of Breathing Therapy on Complaints, Anxiety and Breathing Pattern in Patients with Hyperventilation Syndrome and Anxiety Disorders. J. Psychosom. Res. 1996;41:481–493. doi: 10.1016/S0022-3999(96)00220-6. [DOI] [PubMed] [Google Scholar]
- 23.Banushi B., Brendle M., Ragnhildstveit A., Murphy T., Moore C., Egberts J., Robison R. Breathwork Interventions for Adults with Clinically Diagnosed Anxiety Disorders: A Scoping Review. Brain Sci. 2023;13:256. doi: 10.3390/brainsci13020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyro T.M., Versella M.V., Yang M.-J., Brinkman H.R., Hoyt D.L., Lehrer P. Respiratory Therapy for the Treatment of Anxiety: Meta-Analytic Review and Regression. Clin. Psychol. Rev. 2021;84:101980. doi: 10.1016/j.cpr.2021.101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malviya S., Meredith P., Zupan B., Kerley L. Identifying Alternative Mental Health Interventions: A Systematic Review of Randomized Controlled Trials of Chanting and Breathwork. J. Spiritual. Ment. Health. 2022;24:191–233. doi: 10.1080/19349637.2021.2010631. [DOI] [Google Scholar]
- 26.Ashhad S., Kam K., Del Negro C.A., Feldman J.L. Breathing Rhythm and Pattern and Their Influence on Emotion. Annu. Rev. Neurosci. 2022;45:223–247. doi: 10.1146/annurev-neuro-090121-014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tort A.B.L., Brankačk J., Draguhn A. Respiration-Entrained Brain Rhythms Are Global but Often Overlooked. Trends Neurosci. 2018;41:186–197. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Masaoka Y., Homma I. Anxiety and Respiratory Patterns: Their Relationship during Mental Stress and Physical Load. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 1997;27:153–159. doi: 10.1016/S0167-8760(97)00052-4. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm F.H., Gevirtz R., Roth W.T. Respiratory Dysregulation in Anxiety, Functional Cardiac, and Pain Disorders. Assessment, Phenomenology, and Treatment. Behav. Modif. 2001;25:513–545. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- 30.Martinez J.M., Kent J.M., Coplan J.D., Browne S.T., Papp L.A., Sullivan G.M., Kleber M., Perepletchikova F., Fyer A.J., Klein D.F., et al. Respiratory Variability in Panic Disorder. Depress. Anxiety. 2001;14:232–237. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- 31.Nardi A.E., Freire R.C., Zin W.A. Panic Disorder and Control of Breathing. Respir. Physiol. Neurobiol. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Bechbache R.R., Chow H.H., Duffin J., Orsini E.C. The Effects of Hypercapnia, Hypoxia, Exercise and Anxiety on the Pattern of Breathing in Man. J. Physiol. 1979;293:285–300. doi: 10.1113/jphysiol.1979.sp012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman P. Respiration, Stress, and Cardiovascular Function. Psychophysiology. 1983;20:284–300. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhimani N.T., Kulkarni N.B., Kowale A., Salvi S. Effect of Pranayama on Stress and Cardiovascular Autonomic Function. Indian J. Physiol. Pharmacol. 2011;55:370–377. [PubMed] [Google Scholar]
- 35.Fincham G.W., Strauss C., Montero-Marin J., Cavanagh K. Effect of Breathwork on Stress and Mental Health: A Meta-Analysis of Randomised-Controlled Trials. Sci. Rep. 2023;13:432. doi: 10.1038/s41598-022-27247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopper S.I., Murray S.L., Ferrara L.R., Singleton J.K. Effectiveness of Diaphragmatic Breathing for Reducing Physiological and Psychological Stress in Adults: A Quantitative Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2019;17:1855–1876. doi: 10.11124/JBISRIR-2017-003848. [DOI] [PubMed] [Google Scholar]
- 37.Miri S., Hosseini S.J., Takasi P., Mollaei A., Firooz M., Falakdami A., Osuji J., Ghorbani Vajargah P., Karkhah S. Effects of Breathing Exercise Techniques on the Pain and Anxiety of Burn Patients: A Systematic Review and Meta-Analysis. Int. Wound J. 2023;20:2360–2375. doi: 10.1111/iwj.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rung O., Stauber L., Loescher L.J., Pace T.W. Alternate Nostril Breathing to Reduce Stress: An Option for Pregnant Women Survivors of Intimate Partner Violence? J. Holist. Nurs. Off. J. Am. Holist. Nurses Assoc. 2021;39:393–415. doi: 10.1177/0898010120983659. [DOI] [PubMed] [Google Scholar]
- 39.Aktaş G.K., İlgin V.E. The Effect of Deep Breathing Exercise and 4-7-8 Breathing Techniques Applied to Patients After Bariatric Surgery on Anxiety and Quality of Life. Obes. Surg. 2023;33:920–929. doi: 10.1007/s11695-022-06405-1. [DOI] [PubMed] [Google Scholar]
- 40.Biggs Q.M., Kelly K.S., Toney J.D. The Effects of Deep Diaphragmatic Breathing and Focused Attention on Dental Anxiety in a Private Practice Setting. J. Dent. Hyg. JDH. 2003;77:105–113. [PubMed] [Google Scholar]
- 41.Boaviagem A., Melo Junior E., Lubambo L., Sousa P., Aragão C., Albuquerque S., Lemos A. The Effectiveness of Breathing Patterns to Control Maternal Anxiety during the First Period of Labor: A Randomized Controlled Clinical Trial. Complement. Ther. Clin. Pract. 2017;26:30–35. doi: 10.1016/j.ctcp.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Holmes D.S., McCaul K.D., Solomon S. Control of Respiration as a Means of Controlling Responses to Threat. J. Pers. Soc. Psychol. 1978;36:198–204. doi: 10.1037/0022-3514.36.2.198. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson L.L. Exploring Effects of Therapeutic Massage and Patient Teaching in the Practice of Diaphragmatic Breathing on Blood Pressure, Stress, and Anxiety in Hypertensive African-American Women: An Intervention Study. J. Natl. Black Nurses Assoc. JNBNA. 2010;21:17–24. [PubMed] [Google Scholar]
- 44.Kamath A., Urval R.P., Shenoy A.K. Effect of Alternate Nostril Breathing Exercise on Experimentally Induced Anxiety in Healthy Volunteers Using the Simulated Public Speaking Model: A Randomized Controlled Pilot Study. BioMed Res. Int. 2017;2017:2450670. doi: 10.1155/2017/2450670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin I.M., Tai L.Y., Fan S.Y. Breathing at a Rate of 5.5 Breaths per Minute with Equal Inhalation-to-Exhalation Ratio Increases Heart Rate Variability. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2014;91:206–211. doi: 10.1016/j.ijpsycho.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Meier M., Wirz L., Dickinson P., Pruessner J.C. Laughter Yoga Reduces the Cortisol Response to Acute Stress in Healthy Individuals. Stress. 2021;24:44–52. doi: 10.1080/10253890.2020.1766018. [DOI] [PubMed] [Google Scholar]
- 47.Ratcliff C.G., Prinsloo S., Chaoul A., Zepeda S.G., Cannon R., Spelman A., Yang W.T., Cohen L. A Randomized Controlled Trial of Brief Mindfulness Meditation for Women Undergoing Stereotactic Breast Biopsy. J. Am. Coll. Radiol. JACR. 2019;16:691–699. doi: 10.1016/j.jacr.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Sakakibara M., Hayano J. Effect of Slowed Respiration on Cardiac Parasympathetic Response to Threat. Psychosom. Med. 1996;58:32–37. doi: 10.1097/00006842-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Telles S., Vishwakarma B., Gupta R.K., Balkrishna A. Changes in Shape and Size Discrimination and State Anxiety After Alternate-Nostril Yoga Breathing and Breath Awareness in One Session Each. Med. Sci. Monit. Basic Res. 2019;25:121–127. doi: 10.12659/MSMBR.914956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas M., Bruton A., Little P., Holgate S., Lee A., Yardley L., George S., Raftery J., Versnel J., Price D., et al. A Randomised Controlled Study of the Effectiveness of Breathing Retraining Exercises Taught by a Physiotherapist Either by Instructional DVD or in Face-to-Face Sessions in the Management of Asthma in Adults. Health Technol. Assess. Winch. Engl. 2017;21:1–162. doi: 10.3310/hta21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen K.W., Berger C.C., Manheimer E., Forde D., Magidson J., Dachman L., Lejuez C.W. Meditative Therapies for Reducing Anxiety: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Depress. Anxiety. 2012;29:545–562. doi: 10.1002/da.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaccaro A., Piarulli A., Laurino M., Garbella E., Menicucci D., Neri B., Gemignani A. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front. Hum. Neurosci. 2018;12:353. doi: 10.3389/fnhum.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulus M.P. The Breathing Conundrum–Interoceptive Sensitivity and Anxiety. Depress. Anxiety. 2013;30:315–320. doi: 10.1002/da.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruth D.A. The Health Benefits of Nose Breathing. Nurs. Gen. Pract. 2015:40–42. [Google Scholar]
- 55.Jerath R., Beveridge C., Barnes V.A. Self-Regulation of Breathing as an Adjunctive Treatment of Insomnia. Front. Psychiatry. 2019;9:780. doi: 10.3389/fpsyt.2018.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jerath R., Crawford M.W., Barnes V.A., Harden K. Self-Regulation of Breathing as a Primary Treatment for Anxiety. Appl. Psychophysiol Biofeedback. 2015;40:107–115. doi: 10.1007/s10484-015-9279-8. [DOI] [PubMed] [Google Scholar]
- 57.Ma X., Yue Z.-Q., Gong Z.-Q., Zhang H., Duan N.-Y., Shi Y.-T., Wei G.-X., Li Y.-F. The Effect of Diaphragmatic Breathing on Attention, Negative Affect and Stress in Healthy Adults. Front. Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pal G.K., Velkumary S. Effect of Short-Term Practice of Breathing Exercises on Autonomic Functions in Normal Human Volunteers. Indian J. Med. Res. 2004;120:115–121. [PubMed] [Google Scholar]
- 59.Saoji A.A., Raghavendra B.R., Manjunath N.K. Effects of Yogic Breath Regulation: A Narrative Review of Scientific Evidence. J. Ayurveda Integr. Med. 2019;10:50–58. doi: 10.1016/j.jaim.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sovik R. The Science of Breathing—The Yogic View. Prog. Brain Res. 2000;122:491–505. doi: 10.1016/s0079-6123(08)62159-7. [DOI] [PubMed] [Google Scholar]
- 61.Victoria H.K., Caldwell C. Breathwork in body psychotherapy: Clinical applications. Body Mov. Dance Psychother. 2013;8:216–228. doi: 10.1080/17432979.2013.828657. [DOI] [Google Scholar]
- 62.Boutron I., Moher D., Tugwell P., Giraudeau B., Poiraudeau S., Nizard R., Ravaud P. A Checklist to Evaluate a Report of a Nonpharmacological Trial (CLEAR NPT) Was Developed Using Consensus. J. Clin. Epidemiol. 2005;58:1233–1240. doi: 10.1016/j.jclinepi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Study Quality Assessment Tools|NHLBI, NIH. [(accessed on 17 August 2023)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 64.Mehta C.R., Patel N.R. A Network Algorithm for Performing Fisher’s Exact Test in r × c Contingency Tables. J. Am. Stat. Assoc. 1983;78:427–434. doi: 10.2307/2288652. [DOI] [Google Scholar]
- 65.Fisher R.A. Statistical Methods for Research Workers. In: Kotz S., Johnson N.L., editors. Breakthroughs in Statistics: Methodology and Distribution. Springer; New York, NY, USA: 1992. pp. 66–70. (Springer Series in Statistics). [Google Scholar]
- 66.Khng K.H. A Better State-of-Mind: Deep Breathing Reduces State Anxiety and Enhances Test Performance through Regulating Test Cognitions in Children. Cogn. Emot. 2017;31:1502–1510. doi: 10.1080/02699931.2016.1233095. [DOI] [PubMed] [Google Scholar]
- 67.Bargale S., Khandelwal J.R., Dave B.H., Deshpande A.N., Shah S.S., Chari D.N. Comparative Evaluation of Effect of Two Relaxation Breathing Exercises on Anxiety during Buccal Infiltration Anesthesia in Children Aged 6–12 Years: A Randomized Clinical Study. J. Indian Soc. Pedod. Prev. Dent. 2021;39:284–290. doi: 10.4103/jisppd.jisppd_501_20. [DOI] [PubMed] [Google Scholar]
- 68.Hakked C.S., Balakrishnan R., Krishnamurthy M.N. Yogic Breathing Practices Improve Lung Functions of Competitive Young Swimmers. J. Ayurveda Integr. Med. 2017;8:99–104. doi: 10.1016/j.jaim.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta P.K., Kumar M., Kumari R., Deo J.M. Anuloma-Viloma Pranayama and Anxiety and Depression among the Aged. J. Indian Acad. Appl. Psychol. 2010;1:159–164. [Google Scholar]
- 70.Sharma V.K., Trakroo M., Subramaniam V., Rajajeyakumar M., Bhavanani A.B., Sahai A. Effect of Fast and Slow Pranayama on Perceived Stress and Cardiovascular Parameters in Young Health-Care Students. Int. J. Yoga. 2013;6:104–110. doi: 10.4103/0973-6131.113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundram B.M., Dahlui M., Chinna K. “Taking My Breath Away by Keeping Stress at Bay”–An Employee Assistance Program in the Automotive Assembly Plant. Iran. J. Public Health. 2014;43:263–272. [PMC free article] [PubMed] [Google Scholar]
- 72.Schmalzl L., Powers C., Zanesco A.P., Yetz N., Groessl E.J., Saron C.D. The Effect of Movement-Focused and Breath-Focused Yoga Practice on Stress Parameters and Sustained Attention: A Randomized Controlled Pilot Study. Conscious. Cogn. 2018;65:109–125. doi: 10.1016/j.concog.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Okado Y., De Pace D., Ewing E., Rowley C. Brief Relaxation Training for the Prevention of Stress-Related Difficulties: A Pilot Study. Int. Q. Community Health Educ. 2020;40:193–200. doi: 10.1177/0272684X19873787. [DOI] [PubMed] [Google Scholar]
- 74.Magnon V., Dutheil F., Vallet G.T. Benefits from One Session of Deep and Slow Breathing on Vagal Tone and Anxiety in Young and Older Adults. Sci. Rep. 2021;11:19267. doi: 10.1038/s41598-021-98736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balban M.Y., Neri E., Kogon M.M., Weed L., Nouriani B., Jo B., Holl G., Zeitzer J.M., Spiegel D., Huberman A.D. Brief Structured Respiration Practices Enhance Mood and Reduce Physiological Arousal. Cell Rep. Med. 2023;4:100895. doi: 10.1016/j.xcrm.2022.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busch V., Magerl W., Kern U., Haas J., Hajak G., Eichhammer P. The Effect of Deep and Slow Breathing on Pain Perception, Autonomic Activity, and Mood Processing—An Experimental Study. Pain Med. Malden Mass. 2012;13:215–228. doi: 10.1111/j.1526-4637.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 77.Naik G.S., Gaur G.S., Pal G.K. Effect of Modified Slow Breathing Exercise on Perceived Stress and Basal Cardiovascular Parameters. Int. J. Yoga. 2018;11:53–58. doi: 10.4103/ijoy.IJOY_41_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conlon A., Arnold R., Preatoni E., Moore L.J. Pulling the Trigger: The Effect of a 5-Minute Slow Diaphragmatic Breathing Intervention on Psychophysiological Stress Responses and Pressurized Pistol Shooting Performance. J. Sport Exerc. Psychol. 2022;44:206–219. doi: 10.1123/jsep.2021-0213. [DOI] [PubMed] [Google Scholar]
- 79.Schlatter S.T., Thérond C.C., Guillot A., Louisy S.P., Duclos A., Lehot J.-J., Rimmelé T., Debarnot U.S., Lilot M.E. Effects of Relaxing Breathing Paired with Cardiac Biofeedback on Performance and Relaxation during Critical Simulated Situations: A Prospective Randomized Controlled Trial. BMC Med. Educ. 2022;22:422. doi: 10.1186/s12909-022-03420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark M.E., Hirschman R. Effects of Paced Respiration on Anxiety Reduction in a Clinical Population. Biofeedback Self-Regul. 1990;15:273–284. doi: 10.1007/BF01011109. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y.-F., Huang X.-Y., Chien C.-H., Cheng J.-F. The Effectiveness of Diaphragmatic Breathing Relaxation Training for Reducing Anxiety. Perspect. Psychiatr. Care. 2017;53:329–336. doi: 10.1111/ppc.12184. [DOI] [PubMed] [Google Scholar]
- 82.Serafim S.D., da Ponte F.D.R., Lima F.M., Martins D.S., Rosa A.R., Kunz M. Effects of Deep Breathing in Patients with Bipolar Disorder. Perspect. Psychiatr. Care. 2019;55:119–125. doi: 10.1111/ppc.12325. [DOI] [PubMed] [Google Scholar]
- 83.Clark D.M., Salkovskis P.M., Chalkley A.J. Respiratory Control as a Treatment for Panic Attacks. J. Behav. Ther. Exp. Psychiatry. 1985;16:23–30. doi: 10.1016/0005-7916(85)90026-6. [DOI] [PubMed] [Google Scholar]
- 84.Hayama Y., Inoue T. The Effects of Deep Breathing on “tension-Anxiety” and Fatigue in Cancer Patients Undergoing Adjuvant Chemotherapy. Complement. Ther. Clin. Pract. 2012;18:94–98. doi: 10.1016/j.ctcp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Valenza M.C., Valenza-Peña G., Torres-Sánchez I., González-Jiménez E., Conde-Valero A., Valenza-Demet G. Effectiveness of Controlled Breathing Techniques on Anxiety and Depression in Hospitalized Patients with COPD: A Randomized Clinical Trial. Respir. Care. 2014;59:209–215. doi: 10.4187/respcare.02565. [DOI] [PubMed] [Google Scholar]
- 86.Mobini Bidgoli M., Taghadosi M., Gilasi H., Farokhian A. The Effect of Sukha Pranayama on Anxiety in Patients Undergoing Coronary Angiography: A Single -Blind Randomized Controlled Trial. J. Cardiovasc. Thorac. Res. 2016;8:170–175. doi: 10.15171/jcvtr.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cicek S., Basar F. The Effects of Breathing Techniques Training on the Duration of Labor and Anxiety Levels of Pregnant Women. Complement. Ther. Clin. Pract. 2017;29:213–219. doi: 10.1016/j.ctcp.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Chandrababu R., Kurup S.B., Ravishankar N., Ramesh J. Effect of Pranayama on Anxiety and Pain among Patients Undergoing Cardiac Surgery: A Non-Randomized Controlled Trial. Clin. Epidemiol. Glob. Health. 2019;7:606–610. doi: 10.1016/j.cegh.2019.01.009. [DOI] [Google Scholar]
- 89.Grinberg A.S., Sellinger J.J., Sprenkle P.C., Bandin A.J., Nawaf C.B., Syed J.S., Leapman M.S. Effect of Diaphragmatic Breathing on Procedural Anxiety During Transrectal Prostate Biopsy. Urology. 2020;137:26–32. doi: 10.1016/j.urology.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 90.Abo El Ata A.B., Shaheen E.S., Abdelgilil Khalil A., Yousef Saleh A.M.N. Effect of breathing exercise on reducing pain associated with dressing among hospitalized burned patients. Port Said Sci. J. Nurs. 2021;8:76–102. doi: 10.21608/pssjn.2021.182021. [DOI] [Google Scholar]
- 91.Hosseinzadeh-Karimkoshteh M., Firouzkouhi M., Masinaeinezhad N., Abdollahimohammad A. The Effect of Modified Relaxation Breathing Technique on Pain Anxiety in Patients with Second-Degree Burns: A Clinical Trial Study. J. Nurs. Explor. 2021;1:11–14. doi: 10.29252/jnexp.1.1.11. [DOI] [Google Scholar]
- 92.Zahn C.A., Kuonen F. Deep Breathing Reduces Intraoperative Anxiety and Pain Perception in Patients Undergoing Dermatologic Surgery. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2021;47:1137–1139. doi: 10.1097/DSS.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 93.Moghadam Z.E., Delmoradi F., Aemmi S.Z., Vaghee S., Vashani H.B. Effectiveness of Aromatherapy with Inhaled Lavender Essential Oil and Breathing Exercises on ECT-Related Anxiety in Depressed Patients. Explore. 2022;18:683–687. doi: 10.1016/j.explore.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 94.Dhruva A., Miaskowski C., Abrams D., Acree M., Cooper B., Goodman S., Hecht F.M. Yoga Breathing for Cancer Chemotherapy-Associated Symptoms and Quality of Life: Results of a Pilot Randomized Controlled Trial. J. Altern. Complement. Med. 2012;18:473–479. doi: 10.1089/acm.2011.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park E., Oh H., Kim T. The Effects of Relaxation Breathing on Procedural Pain and Anxiety during Burn Care. Burns J. Int. Soc. Burn Inj. 2013;39:1101–1106. doi: 10.1016/j.burns.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 96.Eldin S.M.A., Mohamed H.G., Ragab I.I. Effect of Relaxation Breathing Technique among Patients with Moderate Burn on Their Pain and Anxiety at Wound Care. World J. Nurs. Sci. 2015;1:110–123. [Google Scholar]
- 97.Iyer A.R., Mitra S., Dabadghav R. The Effect of Rhythmic Deep Breathing on Pain and Anxiety in Patients with Burns. Indian J. Burn. 2020;28:74. doi: 10.4103/ijb.ijb_5_20. [DOI] [Google Scholar]
- 98.Ursavas F., Catakli M. The Effect of Diaphragmatic Breathing Exercise on Pain, Anxiety, and Depression in Patients Undergoing Total Knee Replacement: A Randomized Controlled Trial. Ann. Med. Res. 2020;27:2408. doi: 10.5455/annalsmedres.2020.04.295. [DOI] [Google Scholar]
- 99.Lu H.-B., Liu X., Wang Y.-Q., Cao H.-P., Ma R.-C., Yin Y.-Y., Song C.-Y., Yang T.-T., Xie J. Active Cycle of Breathing Technique: A Respiratory Modality to Improve Perioperative Outcomes in Patients With Lung Cancer. Clin. J. Oncol. Nurs. 2022;26:176–182. doi: 10.1188/22.CJON.176-182. [DOI] [PubMed] [Google Scholar]
- 100.Marshall R.S., Basilakos A., Williams T., Love-Myers K. Exploring the Benefits of Unilateral Nostril Breathing Practice Post-Stroke: Attention, Language, Spatial Abilities, Depression, and Anxiety. J. Altern. Complement. Med. 2014;20:185–194. doi: 10.1089/acm.2013.0019. [DOI] [PubMed] [Google Scholar]
- 101.Thomas M., McKinley R.K., Mellor S., Watkin G., Holloway E., Scullion J., Shaw D.E., Wardlaw A., Price D., Pavord I. Breathing Exercises for Asthma: A Randomised Controlled Trial. Thorax. 2009;64:55–61. doi: 10.1136/thx.2008.100867. [DOI] [PubMed] [Google Scholar]
- 102.Sureka P., Govil S., Dash D., Dash C., Kumar M., Singhal V. Effect of Sudarshan Kriya on Male Prisoners with Non Psychotic Psychiatric Disorders: A Randomized Control Trial. Asian J. Psychiatry. 2014;12:43–49. doi: 10.1016/j.ajp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 103.Sureka P., Govil S., Dash D., Dash C., Kumar C., Singhal V. Sudarshan Kriya for Male Patients with Psycho Active Substance Dependence: A Randomized Control Trial. Asian J. Psychiatry. 2015;16:28–37. [Google Scholar]
- 104.Fiskin G., Sahin N.H. Effect of Diaphragmatic Breathing Exercise on Psychological Parameters in Gestational Diabetes: A Randomised Controlled Trial. Eur. J. Integr. Med. 2018;23:50–56. doi: 10.1016/j.eujim.2018.09.006. [DOI] [Google Scholar]
- 105.Watson G.M., Sutherland J., Lacey C., Bridgman P.G. A Randomised Cross-over Trial of QT Response to Hyperventilation-Induced Anxiety and Diaphragmatic Breathing in Patients with Stress Cardiomyopathy and in Control Patients. PLoS ONE. 2022;17:e0265607. doi: 10.1371/journal.pone.0265607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCaul K.D., Solomon S., Holmes D.S. Effects of Paced Respiration and Expectations on Physiological and Psychological Responses to Threat. J. Pers. Soc. Psychol. 1979;37:564–571. doi: 10.1037/0022-3514.37.4.564. [DOI] [PubMed] [Google Scholar]
- 107.Sharma S.K., Kala N., Telles S. Volitional Yoga Breathing Influences Attention and Anxiety: An Exploratory Randomized Crossover Study. Complement. Med. Res. 2022;29:120–126. doi: 10.1159/000519715. [DOI] [PubMed] [Google Scholar]
- 108.PORGES S.W. The Polyvagal Theory: New Insights into Adaptive Reactions of the Autonomic Nervous System. Cleve. Clin. J. Med. 2009;76:S86–S90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thayer J.F., Lane R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000;61:201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 110.Battaglia M., Ogliari A., D’Amato F., Kinkead R. Early-Life Risk Factors for Panic and Separation Anxiety Disorder: Insights and Outstanding Questions Arising from Human and Animal Studies of CO2 Sensitivity. Neurosci. Biobehav. Rev. 2014;46:455–464. doi: 10.1016/j.neubiorev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Brown R.P., Gerbarg P.L. Sudarshan Kriya Yogic Breathing in the Treatment of Stress, Anxiety, and Depression: Part I-Neurophysiologic Model. J. Altern. Complement. Med. 2005;11:189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- 112.Strohmaier S. The Relationship Between Doses of Mindfulness-Based Programs and Depression, Anxiety, Stress, and Mindfulness: A Dose-Response Meta-Regression of Randomized Controlled Trials. Mindfulness. 2020;11:1315–1335. doi: 10.1007/s12671-020-01319-4. [DOI] [Google Scholar]
- 113.Driscoll D., Dicicco G. The Effects of Metronome Breathing on the Variability of Autonomic Activity Measurements. J. Manip. Physiol. Ther. 2000;23:610–614. doi: 10.1067/mmt.2000.110944. [DOI] [PubMed] [Google Scholar]
- 114.Li C., Chang Q., Zhang J., Chai W. Effects of Slow Breathing Rate on Heart Rate Variability and Arterial Baroreflex Sensitivity in Essential Hypertension. Medicine. 2018;97:e0639. doi: 10.1097/MD.0000000000010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kromenacker B.W., Sanova A.A., Marcus F.I., Allen J.J.B., Lane R.D. Vagal Mediation of Low-Frequency Heart Rate Variability During Slow Yogic Breathing. Psychosom. Med. 2018;80:581–587. doi: 10.1097/PSY.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 116.Lehrer P., Sasaki Y., Saito Y. Zazen and Cardiac Variability. Psychosom. Med. 1999;61:812–821. doi: 10.1097/00006842-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 117.Fiorentini A., Ora J., Tubani L. Autonomic System Modification in Zen Practitioners. Indian J. Med. Sci. 2013;67:161. doi: 10.4103/0019-5359.125877. [DOI] [PubMed] [Google Scholar]
- 118.Abelson J.L., Khan S., Giardino N. HPA Axis, Respiration and the Airways in Stress—A Review in Search of Intersections. Biol. Psychol. 2010;84:57–65. doi: 10.1016/j.biopsycho.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 119.Griffin A. Adolescent Neurological Development and Implications for Health and Well-Being. Healthcare. 2017;5:62. doi: 10.3390/healthcare5040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim H.-G., Cheon E.-J., Bai D.-S., Lee Y.H., Koo B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018;15:235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laborde S., Allen M.S., Borges U., Dosseville F., Hosang T.J., Iskra M., Mosley E., Salvotti C., Spolverato L., Zammit N., et al. Effects of Voluntary Slow Breathing on Heart Rate and Heart Rate Variability: A Systematic Review and a Meta-Analysis. Neurosci. Biobehav. Rev. 2022;138:104711. doi: 10.1016/j.neubiorev.2022.104711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data synthesis and output available in Supplementary Materials. Other data available on request.