The human gut microbiome in critical illness: disruptions, consequences, and therapeutic frontiers (original) (raw)
. Author manuscript; available in PMC: 2024 Apr 22.
Abstract
With approximately 39 trillion cells and over 20 million genes, the human gut microbiome plays an integral role in both health and disease. Modern living has brought a widespread use of processed food and beverages, antimicrobial and immunomodulatory drugs, and invasive procedures, all of which profoundly disrupt the delicate homeostasis between the host and its microbiome. Of particular interest is the human gut microbiome, which is progressively being recognized as an important contributing factor in many aspects of critical illness, from predisposition to recovery. Herein, we describe the current understanding of the adverse impacts of standard intensive care interventions on the human gut microbiome and delve into how these microbial alterations can influence patient outcomes. Additionally, we explore the potential association between the gut microbiome and post-intensive care syndrome, shedding light on a previously underappreciated avenue that may enhance patient recuperation following critical illness. There is an impending need for future epidemiological studies to encompass detailed phenotypic analyses of gut microbiome perturbations. Interventions aimed at restoring the gut microbiome represent a promising therapeutic frontier in the quest to prevent and treat critical illnesses.
Keywords: Dysbiosis, Microbiome, Critical illness, Gut, Fermented foods
“All diseases begin in the gut. Let food be thy medicine.”
–Hippocrates
1. The human gut microbiome and its pivotal relevance to critical illnesses
The human microbiome consists of a complex mix of commensal and pathogenic microorganisms, including bacteria, viruses, fungi, and parasites, with the majority found in the gastrointestinal tract (gut). It is an integral part of who we are as a species: our bodies contain more bacterial than human cells; [1] and for an estimated 20 million bacterial genes we have approximately 20 thousand human genes. [2] This review will concentrate on the growing importance of the human gut bacterial microbiome, aiming to acquaint intensivists with its rapidly expanding impact on various facets of critical illness.
Bacteria are taxonomically categorized into several levels, ranging from phyla to strains. The three dominant phyla in the human gut are Firmicutes (containing over 200 mostly gram-positive genera including Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminococcus); Bacteroidetes (including genera of Bacteroides and Prevotella); and Actinobacteria (with families of Bifidobacteriaceae and Coriobacteriaceae). [3] Our long-standing co-evolution with these organisms has fostered a largely symbiotic relationship: given a hospitable environment and nutrients, the gut microbiome helps to regulate the host’s metabolism and immune system function, modulate enteric and central nervous system activity, support the gut barrier, protect from pathogen invasion, synthesize vitamins and amino acids, and ferment non-digestible fibers to produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate (Fig. 1). [4–6] These SCFAs serve vital functions in our health, including exerting anti-inflammatory, antineoplastic, and antimicrobial effects; regulating gluconeogenesis, lipogenesis, and cholesterol synthesis; promoting blood-tissue barrier integrity and brain function; and modulating the synthesis of neurotransmitters [7,8]. Factors like dietary and lifestyle choices, including the consumption of ultra-processed foods, nicotine use, exposure to antibacterial products, medical interventions, agricultural practices, pollution, and exposure to toxic chemicals, can profoundly disrupt the gut microbiome’s composition and function. [9–13] This disruption to the microbiome, resulting in the loss of beneficial bacteria, expansion of pathogenic strains, and loss of bacterial diversity in the gastrointestinal tract, is known as dysbiosis. [14]
Fig. 1.
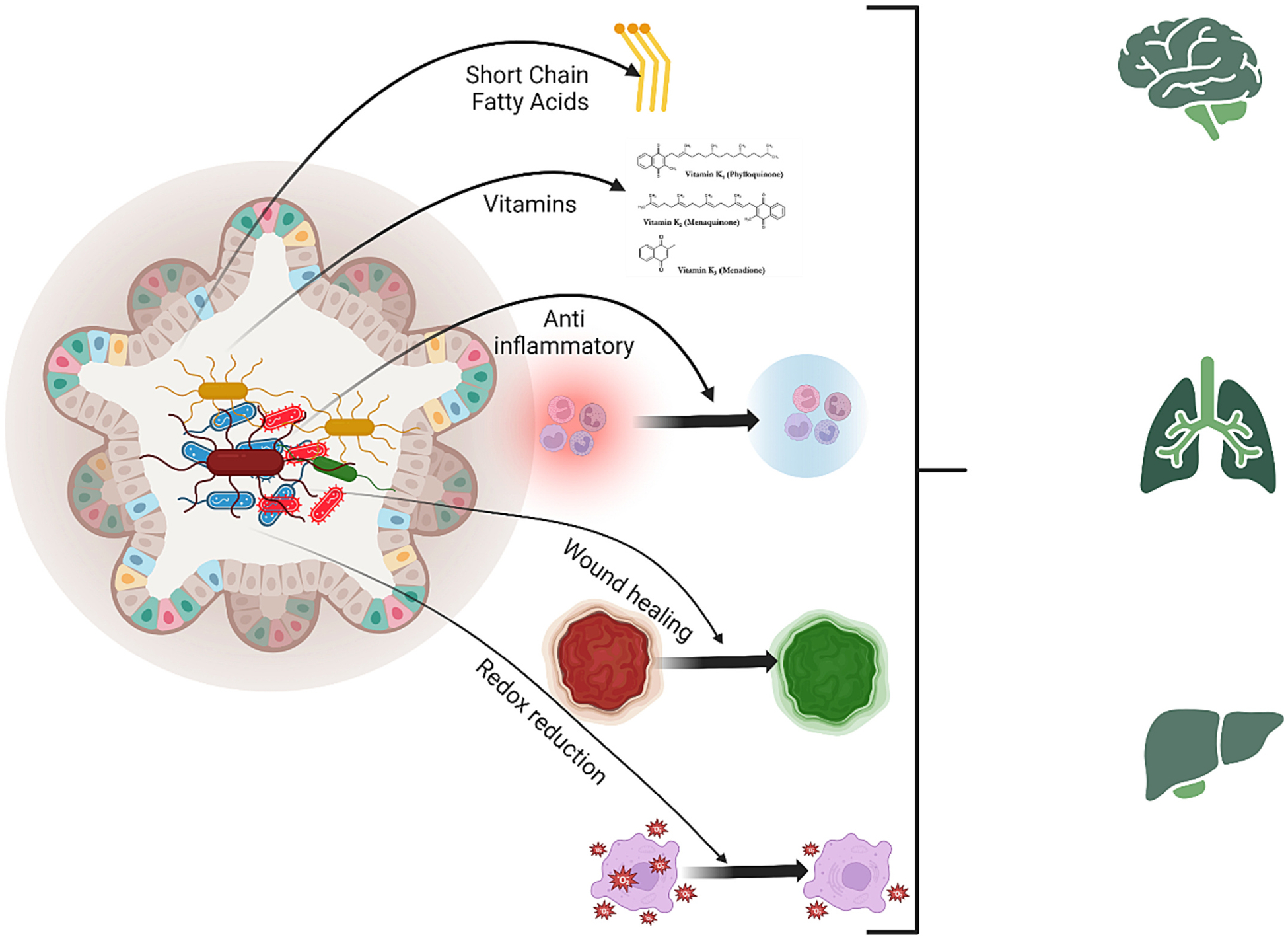
The vital role of the gut microbiome in maintaining health. The gut microbiome plays a crucial role in normal physiological processes, including regulating metabolism, supporting immunity, and fermenting non-digestible fibers to produce SCFAs. It also protects against pathogens, synthesizes vitamins, aids wound healing, and preserves intestinal homeostasis. Alterations in its composition can influence the functions of remote organs such as the brain, lungs, and liver.
Shifts in the gut microbiome’s composition can profoundly influence distant organ systems, playing a pivotal role in the emergence of numerous diseases that frequently result in intensive care unit (ICU) stays. A notable connection exists between gut microbes and lung immunity, termed the “gut-lung axis”. [15] This interaction offers insights into challenges like refractory asthma and increased vulnerability to both viral and community-spread pneumonia. A standout discovery in this realm is that the microbial metabolite desaminotyrosine has been shown to fortify defenses against influenza. [16–19]
The heart isn’t exempt from the influence of our gut residents. The “gut-heart axis” [20] suggests that microbial metabolite imbalances in the gut can play a key role in atherogenesis. This imbalance can initiate a cascade of events: inflammation, disruption of tight cellular junctions, elevated intestinal permeability, and subsequent translocation of lipopolysaccharide (LPS) from the gut into the bloodstream. These events could foster the emergence of cardiovascular complications, heart failure, and elevate risks like ischemic stroke, severe cardiac incidents, and overall worsened outcomes. [21–24]
The “gut-kidney axis” [25] tells a similar story where microbial imbalances are linked to kidney-related ailments. A noteworthy observation is the identification of reduced butyrate-producing bacteria as a leading cause behind type 2 diabetes onset. [26] Furthermore, an overgrowth of specific pathogenic microbes, including the likes of Staphylococcus, Pseudomonas, and Escherichia coli, can culminate in severe conditions like sepsis, peritonitis, and other gut infections. [26,27] Associations have also been drawn between gut microbiome irregularities and a range of gastrointestinal and liver disorders. [28–33]
While the connections between gut microbiome dysbiosis and critical illnesses mentioned above are compelling, they are not yet definitive. Thus, there’s a pressing need for prospective studies that investigate the microbiome’s state before the emergence of critical illnesses, where the aim would be to ascertain if dysbiosis genuinely acts as a risk precursor. As it stands, our current understanding, though intriguing, remains largely circumstantial and speculative.
2. Influence of ICU therapies on gut dysbiosis: Implications for clinical outcomes in critically ill patients
Over 90% of commensal microorganisms are lost within the first 6 h of critical illness [34,35] due to both the disease itself and the treatments one may receive. The release of proinflammatory cytokines into the systemic circulation triggers changes in the tight junction proteins of the gut, leading to hyperpermeability. In turn, this results in bacterial translocation, misregulated immune system activation, inflammation, heightened apoptosis (particularly of intestinal and pulmonary epithelial cells), and a shift in the microbiome population towards more virulent and pathogenic bacteria. [36–38] Factors such as a catabolic state, glucose and electrolyte imbalances, and hypoperfusion further exacerbate intestinal dysmotility and dysbiosis. [39,40] Normally, commensal organisms reside in the crypts of the colonic epithelium, which serve as a reservoir to repopulate the gut microbiome post-illness; however, the combined effects of starvation, antibiotics, and oxidative stress, which are prevalent in ICU settings, might entirely deplete these niches of the symbiotic microbiome. [35] (Fig. 2).
Fig. 2.
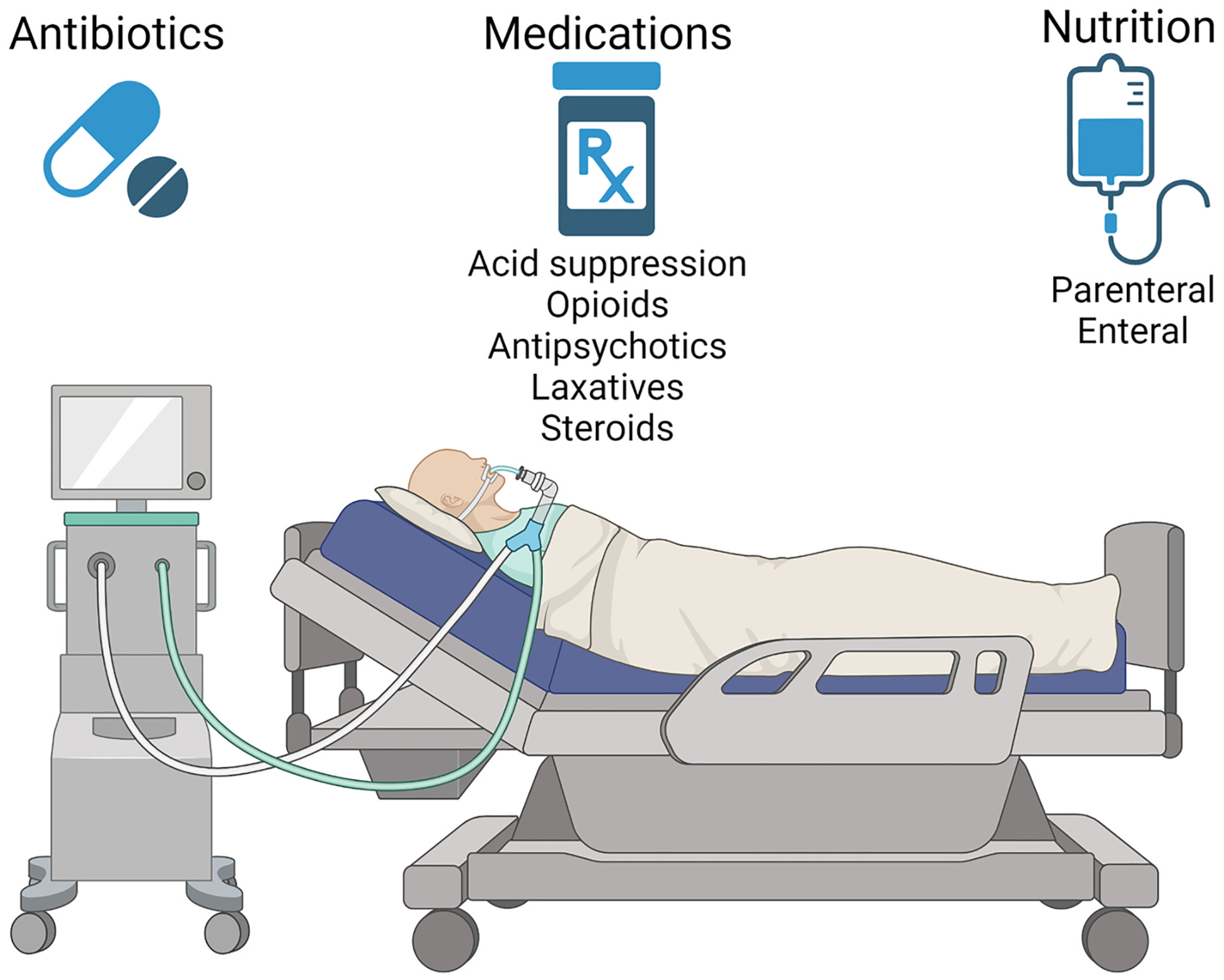
Common therapeutic interventions in critical care medicine that promote gut microbiome dysbiosis. These standard medical practices can substantially reduce gut microbial diversity, predispose ICU patients to infections like C. difficile, select for antibiotic-resistant strains, and potentially result in complications such as muscle loss and an elevated risk of sepsis. This highlights the need for a balanced approach to patient care to prevent inadvertently compromising the gut health of the patient.
2.1. Unintended roles of ICU therapeutic interventions on gut microbiome homeostasis
The overwhelming benefits of antibiotics for critically ill patients in the ICU with an infectious process cannot be overstated. [41] However, antibiotics can also rapidly reduce gut microbial diversity and select for antibiotic-resistant bacterial strains, thereby making the host more susceptible to infection with pathogens such as Clostridium difficile. [42] Additionally, antibiotics can impact the transcription of functional genes involved in metabolism of carbohydrates and protein synthesis [43], potentially leading to the downregulation of SCFA production. This could, in turn, contribute to the profound muscle loss observed in ICU patients. [42,44] A recent retrospective single-center cohort study involving 3032 critically ill patients revealed that an early administration of antibiotics with anaerobic coverage correlated with decreased survival in patients with ventilator-associated pneumonia. [45] The use of antibiotics with broad microbiota-disruptive capabilities has also been linked to an increased risk of sepsis within 90 days of discharge. [46] Yet of the more than 70% of patients who receive antibiotics in the ICU, a notable 25% lack culture- or imaging-confirmed infection. [47] As the rise in antimicrobial resistance is a silent epidemic, the non-judicious use of antimicrobials - in addition to impacting and damaging the gut microbiome - could accelerate the rate at which bacterial species develop resistance.
The administration of gastric acid suppression agents has been associated with a significant increase in Enterococcus, Streptococcus, Staphylococcus, and E. coli, which in turn predisposes individuals to C. difficile infections. [48] Remarkably, even a single day of morphine treatment resulted in an increase in pathogenic bacterial communities and expansion of Enterococcus faecalis, while concurrently decreasing communities associated with stress tolerance. [49] Antipsychotic medications might lead to a reduction in microbiome diversity; interestingly, the bioavailability of these drugs could be influenced by gut microbiome composition, potentially explaining variabilities in patient response. [50,51]
Parenteral nutrition has been observed to elevate levels of Proteo-bacteria, which are known for inciting inflammation at the mucosal level, consequently compromising the epithelial barrier’s integrity. [52] Moreover, enteral nutrition preparations in the ICU are often designed without the gut microbiome in mind; typical refined formulas are absorbed very proximally in the gastrointestinal tract and contain a limited quantity of soluble fibers. [35] Addition of emulsifiers, such as carboxymethylcellulose or polysorbate-80, to these formula can result in the thinning of the mucus layer, reduced SCFA production, and intestinal inflammation. [35] Meanwhile, the use of laxatives can diminish microbiome diversity and weaken the mucus barrier. [53] Such interventions frequently lead clinicians to request diagnostic tests for potential C. difficile infections, which may inadvertently detect colonizing C. difficile, culminating in a positive diagnosis and additional antimicrobial treatments. High-dose prednisone usage has been linked to increased bacterial translocation and a hindered ability to eliminate translocated E. coli. [54] These are but a few examples of how our best medical practices for ICU patients might inadvertently yield unintended adverse outcomes on the gut microbiome.
2.2. Impact of gut dysbiosis on the clinical manifestations and outcomes in critically ill patients
Which organ systems may be affected by dysbiosis? The short answer is likely all of them. While gut microbiome has not traditionally been at the forefront of critical illness-related research, emerging evidence may result in a paradigm shift in the near future. The gut-brain-axis concept is now broadly acknowledged within the scientific community. [55] Individuals with neurocritical conditions demonstrate a markedly distinct gut microbiota composition compared to healthy cohorts, which in turn influences their mortality outcomes. [56] An abnormal gut microbiome composition has been linked to delirium and septic encephalopathy; notably, a resolution of delirium was documented in a patient diagnosed with C. difficile infection post fecal microbiota transplantation (FMT). [57–60] Such microbial imbalances have also been correlated with mild cognitive impairments, dementia, and the behavioral manifestations of depression, schizophrenia, and addiction. [61]
Evaluation of microbial composition of patients with acute respiratory distress syndrome and sepsis revealed the presence of 86 over-lapping species in lung and gut. In particular, an abundance of Enterococcus faecium is thought to occur via translocation; and the resulting dysbiosis has also been associated with mortality among patients undergoing mechanical ventilation. [62,63] Interestingly, bronchoalveolar lavage fluid sampled from individuals with acute respiratory distress syndrome revealed a pronounced presence of gut-specific bacteria (Bacteroides) that remained undetectable via traditional culturing methods, yet exhibited a correlation with systemic inflammatory responses. [64]
Moreover, the gut microbiome is now known to promote the capture and eradication of circulating pathogens by Kupffer cells in vivo, thus protecting against pathogen dissemination during infection. [65] The gut microbiome may also substantially influence the host’s resilience and immune responses during sepsis events, affecting critical parameters including body temperature regulation (a known prognostic indicator) and susceptibility to nosocomial infections and severe sepsis. [66–69] Furthermore, several mechanisms tying intestinal flora to the onset of septic myopathy have been proposed. [70] Notably, the presence of Enterococcus upon ICU admission was associated with risks of death and all-cause infection. [71] Consequently, while dysbiosis is often perceived as a downstream effect of critical illnesses, it may concurrently modulate the host’s physiological responses to such conditions and even serve as a predictor for in-hospital mortality. [72,73]
3. Gut microbiome and post-critical illness recovery
Resolution of critical illness does not equate with the end of a patient’s struggles. The consequences of critical illness may persist long after the ICU stay and manifest in a number of new or worsening impairments in physical, cognitive, or mental health, collectively termed “post-intensive care syndrome” or PICS. [74] Such impairments may persist for years [75–77] and lead to increased re-hospitalization, health care costs, impaired quality of life, and inability to return to work. [78,79] Conventional strategies for facilitating patient recovery focus on specific impairments, often necessitating referrals to specialists possessing the requisite expertise in these areas. [80] Assessments conducted within the PICS clinic may include the following: screening spirometry, a six-minute walk test, medication reconciliation and counseling, a review of the patient’s ICU course and related active medical problems, screening for depression, anxiety, and post-traumatic stress disorder, brief cognitive evaluation, targeted psychotherapy, and targeted case management assessment. [81–83] While these interventions undoubtedly hold significant value and represent an important step in post-ICU follow-up care, are they sufficient and comprehensive enough to optimize recovery? Could addressing gut microbiome changes offer another avenue to further promote clinical recovery?
3.1. Gut-brain-muscle connections in critical illness recovery
The recognition of the intricate communication between the nervous system and the gastrointestinal tract, facilitated through a bidirectional network of signaling pathways termed the gut-brain axis, has culminated in the birth of the burgeoning field of “nutritional psychiatry.” [84] Given that conventional interventions like pharmacotherapy and psychotherapy effectively manage merely half of the mental health disease burden, [85] the field of nutritional psychiatry has positioned diet quality and nutrition as central determinants of mental health. [84] The fact that the gut microbiome may play a crucial role in mood symptoms is not surprising, when considering that intestinal enterochromaffin cells produce 90% of the body’s serotonin. [86] Additionally, gut bacteria have been identified as producers of a variety of other neurotransmitters, including dopamine, norepinephrine, gamma-aminobutyric acid, and acetylcholine. [87]
While the phrase “_mens sana in corpore sano_”, translating to “_a healthy mind in a healthy body_” has resonated across cultures for nearly two millennia—with variations even predating Socratic thought—systematic research exploring the interconnection between diet, the microbiome, and mood disorders is a relatively recent development. Contemporary studies have identified potential associations between anxiety and depression symptoms with an overabundance of proinflammatory bacteria such as Enterobacteriaceae and Desulfovibrio, and a decrease in SCFA-producing bacteria like Faecalibacterium. [88] The Mediterranean diet has been linked to improvements in mood and depressive symptoms [89,90] possibly via its known anti-inflammatory properties. [91] Contrarily, diets high in sugars and refined grains, known for their high inflammatory potential, [92] have been associated with the onset of depression and cognitive decline. [93–96] In states of dysbiosis, the regulation of gut-brain pathways falters, possibly leading to alterations in the permeability of the blood-brain barrier and consequent neuroinflammation [97]. Such disruptions can manifest as heightened stress reactivity, tendencies towards anxiety and depressive-like behaviors, and cognitive dysfunction. [97–100]
Muscle weakness, recognized as another component of PICS, may also be tied to the gut microbiome through the gut-muscle axis. Specifically, the gut microbiome has a recognized role in modulating the bioavailability of amino acids. [101] Perturbations in the gut microbiota composition can precipitate skeletal muscle atrophy through a bile acid-farnesoid X receptor-mediated pathway. [102] Moreover, the activation of toll-like receptor signaling cascades culminates in the inhibition of muscle mass accrual and the facilitation of muscle atrophy; [103] intriguingly, such activation can be triggered by the translocation of gut microbes or their metabolic products, into the systemic circulation. [104]
It therefore appears that the state of the gut microbiome can profoundly contribute to the major symptoms experienced by survivors of critical illness. What implications does this knowledge hold for shaping prospective therapeutic strategies?
3.2. Restoring the gut microbiome through probiotics and fermented foods
Dysbiosis resulting from critical illness and related therapeutic interventions has been reported to persist even after the resolution of the primary ailment. For example, significant decreases in microbial diversity and anti-inflammatory bacteria have been observed in survivors of acute respiratory distress related to COVID-19, persisting for at least six months following hospital discharge. [105–108] Others reported incomplete microbiome recovery following antibiotic use up to two years later. [109,110] Evaluation of hematopoietic stem cell transplantation survivors revealed significantly decreased Bacteroidetes genera, which conventionally degrade indigestible dietary fibers. [111] A decline in fiber-degrading Bacteroides and Firmicutes, as well as in anti-inflammatory Faecalibacterium species, was also observed in those recuperating from critical illnesses. [112] Others reported that dietary interventions rich in soluble fiber were less effective in ameliorating inflammatory markers in individuals with diminished microbiome richness and that a high-fiber diet alone did not enhance microbial community diversity. [113,114] Given this context, one must ponder: could the re-establishment of microbiome diversity alleviate the psychological, cognitive, and physical repercussions faced by survivors of critical illnesses? If so, what avenues could be explored to achieve this?
Predominantly, clinical trials exploring dysbiosis management in critical care populations have harnessed probiotic supplementation in a bid to modulate the microbiome. The objective has been twofold: to curb the proliferation of pathogenic bacteria and to fortify immune system responsiveness. Notwithstanding these objectives, tangible clinical benefits have remained elusive in many instances. [115,116] One possible explanation for the muted clinical outcomes from these supplementation trials might reside in the observation that probiotic supplementation does not invariably bring about alterations in the microbiota composition or diversity. In some instances, it might even hinder the recovery of the native commensal microbiome. [117–119]
Historically, the consumption of a diet replete with probiotic fermented foods—essentially those bearing live commensal bacterial cultures—has been lauded as a health-sustaining practice. The exemplar being the perceived enhanced longevity of Bulgarian peasants, often attributed to the salubrious effects of lactic acid-producing bacteria found in their fermented milk. [120] In a related vein, a diet containing probiotic fermented foods increased microbiome diversity in healthy volunteers and decreased their inflammatory markers. [114] For instance, yogurt containing the probiotic Bifidobacterium lactis BB-12 outperformed a placebo in preserving the commensal bacterial community within the colon of healthy subjects. This was evident in post-antibiotic therapy, which had initially attenuated fecal acetate levels across both groups. Following the discontinuation of antibiotics, fecal acetate levels in the probiotic group increased over the remainder of the study and returned to the baseline levels on day 30, whereas, in the control group, the acetate levels remained suppressed. [121]
Dietary inclusion of fermented foods has been associated with both an augmented immune response [122–124] and an array of positive health outcomes. Examples include the facilitation of gut homeostasis in irritable bowel syndrome patients and enhancements in subjective well-being (“feeling good”) metrics. [125,126] Interventional studies of fermented tea, sauerkraut, fermented plant extract, kimchi, and fermented soybean milk have all evidenced an uptick in the presence of gut bacteria conducive to health. [127–132] Furthermore, dietary integration of fermented food products has been correlated with favorable modulations in cerebral activity [133] and exhibits a broader neuroprotective influence. [134,135] A comprehensive schematic delineating the proposed restoration of the gut microbiome in survivors of critical illness is presented in Fig. 3.
Fig. 3.
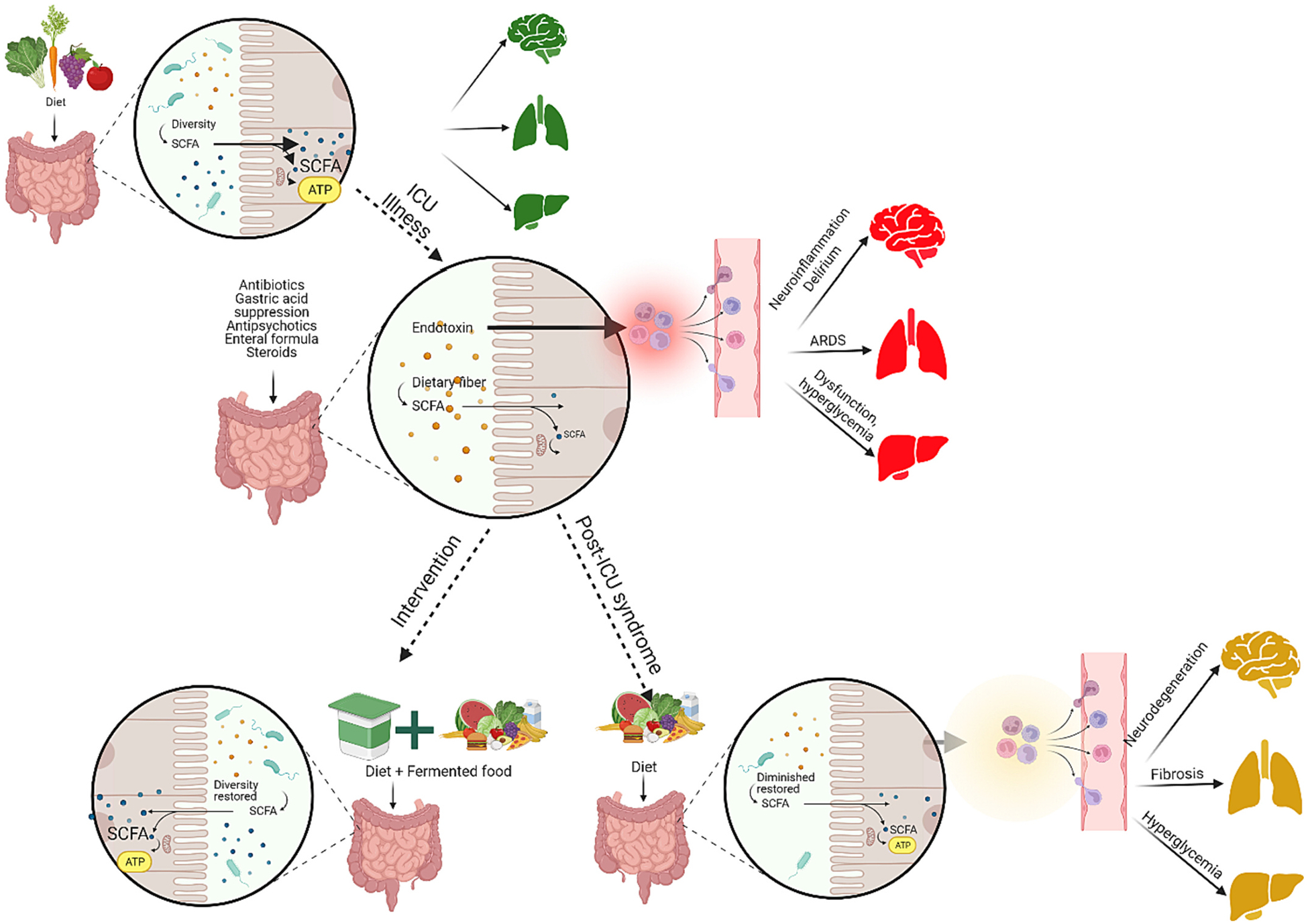
Alterations in the gut microbiome can significantly influence both the progression of disease and the trajectory of recovery in critically ill patients. Those with critical illnesses often exhibit diminished gut microbiome compositions in the ICU, a result of both the underlying disease and the treatments administered. Notably, these microbiome shifts can affect distant organ systems via the gut-brain, gut-lung, and gut-liver axes. Therapeutic interventions designed with the gut microbiome in mind, such as the inclusion of fermented foods, might offer promise in restoring gut health and aiding recovery from critical illnesses and post-intensive care syndrome.
4. Conclusion
Alterations in the gut microbiome are frequently observed in critically ill patients and may significantly influence the progression of the disease and the trajectory of recovery. Comprehensive phenotyping, coupled with extensive observational studies and well-designed randomized clinical trials, will elucidate the implications of these gut microbiome shifts in the context of critical illnesses. This knowledge holds the potential for unveiling novel therapeutic avenues for both prevention and treatment. Historically, prior to the advent of pasteurization, fermentation served as a primary method of food preservation for millennia. [136] Since then, there has been a marked increase in the consumption of processed and ultra-processed foods, with such foods accounting for over two-thirds of calorie intake in the United States. Considering the deleterious impact of these dietary trends on the gut microbiome, [12,13] one might postulate: could the future of critical care, including critical illness prevention, hinge on a re-embracement of the nutritional practices of the past?
Funding
Dr. Karnatovskaia is supported by a grant from the National Heart, Lung, and Blood Institute K23HL146741-1.
Abbreviations:
SCFA
Short-chain Fatty Acid
ICU
Intensive Care Unit
LPS
Lipopolysaccharide
PICS
Post-Intensive Care Syndrome
Footnotes
Financial disclosures and conflicts of interest for each of the authors: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Parts of this manuscript have been presented at the ISICEM “ICU of the Future” 2022 meeting in Rome, Italy.
References
- [1].Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164(3):337–40. [DOI] [PubMed] [Google Scholar]
- [2].Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr Opin Crit Care 2016;22(4):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019:7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J 2015; 39(3):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, et al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol 2018;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. Bmj 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cătoi AF, Corina A, Katsiki N, Vodnar DC, Andreicuț AD, Stoian AP, et al. Gut microbiota and aging-a focus on centenarians. Biochim Biophys Acta Mol basis Dis 1866;2020(7):165765. [DOI] [PubMed] [Google Scholar]
- [8].Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- [9].Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, et al. Unhealthy lifestyle and gut Dysbiosis: a better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne) 2021;12:667066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: where chemistry meets biology. Science 2020;367(6476):392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555(7695):210–5. [DOI] [PubMed] [Google Scholar]
- [12].Atzeni A, Martínez M, Babio N, Konstanti P, Tinahones FJ, Vioque J, et al. Association between ultra-processed food consumption and gut microbiota in senior subjects with overweight/obesity and metabolic syndrome. Front Nutr 2022;9:976547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martínez Leo EE, Segura Campos MR. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition 2020;71: 110609. [DOI] [PubMed] [Google Scholar]
- [14].Belizário JE, Faintuch J. Microbiome and gut Dysbiosis. In: Silvestre R, Torrado E, editors. Metabolic interaction in infection. Cham: Springer International Publishing; 2018. p. 459–76. [DOI] [PubMed] [Google Scholar]
- [15].Rastogi S, Mohanty S, Sharma S, Tripathi P. Possible role of gut microbes and host’s immune response in gut-lung homeostasis. Front Immunol 2022;13: 954339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 2019;7(10):907–20. [DOI] [PubMed] [Google Scholar]
- [17].Fuentes S, den Hartog G, Nanlohy NM, Wijnands L, Ferreira JA, Nicolaie MA, et al. Associations of faecal microbiota with influenza-like illness in participants aged 60 years or older: an observational study. Lancet Healthy Longev 2021;2(1): e13–23. [DOI] [PubMed] [Google Scholar]
- [18].Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: new evidence. J Immunol Res 2020;2020:2340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017;357(6350):498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen W, Zhang S, Wu J, Ye T, Wang S, Wang P, et al. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta 2020;507:236–41. [DOI] [PubMed] [Google Scholar]
- [21].Novakovic M, Rout A, Kingsley T, Kirchoff R, Singh A, Verma V, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol 2020;12(4):110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to Mediterranean Diet. J Am Heart Assoc 2017:6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang Y, Woo HG, Jeong JH, Kim GH, Park KD, Song TJ. Microbiota dysbiosis and functional outcome in acute ischemic stroke patients. Sci Rep 2021;11(1): 10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].CN Lledos M, Carcel-Marquez J, Muino E, Gallego-Fabrega C, Llucia-Carol L, Martin-Campos JM, et al. Influence of the gut microbiome in ischemic stroke risk and ischemic stroke outcome. Lyon, France: ESOC; 2022. [Google Scholar]
- [25].Stavropoulou E, Kantartzi K, Tsigalou C, Konstantinidis T, Romanidou G, Voidarou C, et al. Focus on the gut-kidney axis in health and disease. Front Med (Lausanne) 2020;7:620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iacob S, Iacob DG. Infectious threats, the intestinal barrier, and its Trojan horse: Dysbiosis. Front Microbiol 2019;10:1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, et al. Pretreatment of mice with streptomycin provides a salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 2003;71(5):2839–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11(1): 1–10. [DOI] [PubMed] [Google Scholar]
- [29].Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 2010;1(3):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nishikawa H, Fukunishi S, Asai A, Yokohama K, Ohama H, Nishiguchi S, et al. Dysbiosis and liver diseases (Review). Int J Mol Med 2021:48(3). [DOI] [PubMed] [Google Scholar]
- [31].Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 2015;39(5):763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, et al. Review article: alcohol and gut microbiota - the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther 2015;41(10):917–27. [DOI] [PubMed] [Google Scholar]
- [33].Khan A, Ding Z, Ishaq M, Bacha AS, Khan I, Hanif A, et al. Understanding the effects of gut microbiota Dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci 2021;17(3):818–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Otani S, Chihade DB, Coopersmith CM. Critical illness and the role of the microbiome. Acute Med Surg 2019;6(2):91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McClave SA, Martindale RG. Why do current strategies for optimal nutritional therapy neglect the microbiome? Nutrition 2019;60:100–5. [DOI] [PubMed] [Google Scholar]
- [36].Zhou Q, Verne GN. Intestinal hyperpermeability: a gateway to multi-organ failure? J Clin Invest 2018;128(11):4764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shea-Donohue T, Fasano A, Smith A, Zhao A. Enteric pathogens and gut function: role of cytokines and STATs. Gut Microbes 2010;1(5):316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: the gut in stress. Clin Nutr 2018;37(1):19–36. [DOI] [PubMed] [Google Scholar]
- [39].Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016;4(1): 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Adedeji WA. The treasure called antibiotics. Ann Ib Postgrad Med 2016;14(2): 56–7. [PMC free article] [PubMed] [Google Scholar]
- [42].Partida-Rodríguez O, Serrano-Vázquez A, Nieves-Ramírez ME, Moran P, Rojas L, Portillo T, et al. Human intestinal microbiota: interaction between parasites and the host immune response. Arch Med Res 2017;48(8):690–700. [DOI] [PubMed] [Google Scholar]
- [43].Goh EB, Yim G, Tsui W, McClure J, Surette MG, Davies J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci U S A 2002;99(26):17025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qiu Y, Yu J, Li Y, Yang F, Yu H, Xue M, et al. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann Med 2021;53(1): 508–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chanderraj R, Baker JM, Kay SG, Brown CA, Hinkle KJ, Fergle DJ, et al. In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur Respir J 2023;61(2):2200910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent Sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 2018;66(7):1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wunderink RG, Srinivasan A, Barie PS, Chastre J, Dela Cruz CS, Douglas IS, et al. Antibiotic stewardship in the intensive care unit. An official American Thoracic Society workshop report in collaboration with the AACN, CHEST, CDC, and SCCM. Ann Am Thorac Soc 2020;17(5):531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65(5):740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 2018;8(1):3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tomizawa Y, Kurokawa S, Ishii D, Miyaho K, Ishii C, Sanada K, et al. Effects of Psychotropics on the microbiome in patients with depression and anxiety: considerations in a naturalistic clinical setting. Int J Neuropsychopharmacol 2021;24(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cussotto S, Walsh J, Golubeva AV, Zhdanov AV, Strain CR, Fouhy F, et al. The gut microbiome influences the bioavailability of olanzapine in rats. EBioMedicine 2021;66:103307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Demehri FR, Barrett M, Teitelbaum DH. Changes to the intestinal microbiome with parenteral nutrition: review of a murine model and potential clinical implications. Nutr Clin Pract 2015;30(6):798–806. [DOI] [PubMed] [Google Scholar]
- [53].Tropini C, Moss EL, Merrill BD, Ng KM, Higginbottom SK, Casavant EP, et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018;173(7). 1742–54.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gianotti L, Alexander JW, Fukushima R, Pyles T. Steroid therapy can modulate gut barrier function, host defense, and survival in thermally injured mice. J Surg Res 1996;62(1):53–8. [DOI] [PubMed] [Google Scholar]
- [55].Appleton J The gut-brain axis: influence of microbiota on mood and mental health. Integr Med (Encinitas) 2018;17(4):28–32. [PMC free article] [PubMed] [Google Scholar]
- [56].Xu R, Tan C, Zhu J, Zeng X, Gao X, Wu Q, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care 2019;23 (1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang J, Bi JJ, Guo GJ, Yang L, Zhu B, Zhan GF, et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci Ther 2019;25(6):685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gotoh K, Sakaguchi Y, Kato H, Osaki H, Jodai Y, Wakuda M, et al. Fecal microbiota transplantation as therapy for recurrent Clostridioides difficile infection is associated with amelioration of delirium and accompanied by changes in fecal microbiota and the metabolome. Anaerobe 2022;73:102502. [DOI] [PubMed] [Google Scholar]
- [59].Barlow B, Ponnaluri S, Barlow A, Roth W. Targeting the gut microbiome in the management of sepsis-associated encephalopathy. Front Neurol 2022;13:999035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Garcez FB, Garcia de Alencar JC, Fernandez SSM, Avelino-Silva VI, Sabino EC, Martins RCR, et al. Association between gut microbiota and delirium in acutely ill older adults. J Gerontol A Biol Sci Med Sci 2023;78(8):1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Luca M, Chattipakorn SC, Sriwichaiin S, Luca A. Cognitive-behavioural correlates of dysbiosis: A review. Int J Mol Sci 2020;21(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu B, Yu Y, Zhao M, Xiao K, Yan P, Duan Z, et al. Correlation analysis of the microbiome and immune function in the lung-gut axis of critically ill patients in the ICU. Front Med (Lausanne) 2022;9:808302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lamarche D, Johnstone J, Zytaruk N, Clarke F, Hand L, Loukov D, et al. Microbial dysbiosis and mortality during mechanical ventilation: a prospective observational study. Respir Res 2018;19(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 2016;1(10): 16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McDonald B, Zucoloto AZ, Yu IL, Burkhard R, Brown K, Geuking MB, et al. Programing of an intravascular immune firewall by the gut microbiota protects against pathogen dissemination during infection. Cell Host Microbe 2020;28(5). 660–8.e4. [DOI] [PubMed] [Google Scholar]
- [66].Bongers KS, Chanderraj R, Woods RJ, McDonald RA, Adame MD, Falkowski NR, et al. The gut microbiome modulates body temperature both in sepsis and health. Am J Respir Crit Care Med 2023;207(8):1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fay KT, Klingensmith NJ, Chen CW, Zhang W, Sun Y, Morrow KN, et al. The gut microbiome alters immunophenotype and survival from sepsis. FASEB J 2019;33 (10):11258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schlechte J, Zucoloto AZ, Yu IL, Doig CJ, Dunbar MJ, McCoy KD, et al. Dysbiosis of a microbiota-immune metasystem in critical illness is associated with nosocomial infections. Nat Med 2023;29(4):1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe Sepsis. Am J Respir Crit Care Med 2015;192(5): 581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu Y, Xu L, Yang Z, Wang D, Li T, Yang F, et al. Gut-muscle axis and sepsis-induced myopathy: the potential role of gut microbiota. Biomed Pharmacother 2023;163:114837. [DOI] [PubMed] [Google Scholar]
- [71].Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso DI, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med 2018;44 (8):1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Martin-Loeches I, Dickson R, Torres A, Hanberger H, Lipman J, Antonelli M, et al. The importance of airway and lung microbiome in the critically ill. Crit Care 2020;24(1):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wei R, Chen X, Hu L, He Z, Ouyang X, Liang S, et al. Dysbiosis of intestinal microbiota in critically ill patients and risk of in-hospital mortality. Am J Transl Res 2021;13(3):1548–57. [PMC free article] [PubMed] [Google Scholar]
- [74].Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012;40(2):502–9. [DOI] [PubMed] [Google Scholar]
- [75].Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364(14):1293–304. [DOI] [PubMed] [Google Scholar]
- [77].Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med 2011;39(2):371–9. [DOI] [PubMed] [Google Scholar]
- [78].Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185(12):1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Davydow DS, Hough CL, Zatzick D, Katon WJ. Psychiatric symptoms and acute care service utilization over the course of the year following medical-surgical ICU admission: a longitudinal investigation*. Crit Care Med 2014;42(12):2473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].SCCM. Post-intensive Care Syndrome. https://www.sccm.org/MyICUCare/THRIVE/Post-intensive-Care-Syndrome;; 2022. [accessed accessed November 18, 2022].
- [81].Sevin CM, Bloom SL, Jackson JC, Wang L, Ely EW, Stollings JL. Comprehensive care of ICU survivors: development and implementation of an ICU recovery center. J Crit Care 2018;46:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang S, Hanneman P, Xu C, Gao S, Allen D, Golovyan D, et al. Critical care recovery center: a model of agile implementation in intensive care unit (ICU) survivors. Int Psychogeriatr 2020;32(12):1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs 2015;115(3):24–31. quiz 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015;2(3):271–4. [DOI] [PubMed] [Google Scholar]
- [85].Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 2002;159(8):1354–60. [DOI] [PubMed] [Google Scholar]
- [86].Shah PA, Park CJ, Shaughnessy MP, Cowles RA. Serotonin as a mitogen in the gastrointestinal tract: revisiting a familiar molecule in a new role. Cell Mol Gastroenterol Hepatol 2021;12(3):1093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Strandwitz P Neurotransmitter modulation by the gut microbiota. Brain Res 2018;1693(Pt B):128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - a systematic review. Clin Psychol Rev 2021;83:101943. [DOI] [PubMed] [Google Scholar]
- [89].Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci 2019;22(7):474–87. [DOI] [PubMed] [Google Scholar]
- [91].Firth J, Veronese N, Cotter J, Shivappa N, Hebert JR, Ee C, et al. What is the role of dietary inflammation in severe mental illness? A Review of Observational and Experimental Findings. Front Psychiatry 2019;10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian longitudinal study on Women’s health. Br J Nutr 2016;116(6): 1077–86. [DOI] [PubMed] [Google Scholar]
- [93].Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord 2018;226: 346–54. [DOI] [PubMed] [Google Scholar]
- [94].Akbaraly T, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, et al. Dietary inflammatory index and recurrence of depressive symptoms: results from the Whitehall II study. Clin Psychol Sci 2016;4(6):1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Agarwal P, Dhana K, Barnes LL, Holland TM, Zhang Y, Evans DA, et al. Unhealthy foods may attenuate the beneficial relation of a Mediterranean diet to cognitive decline. Alzheimers Dement 2021;17(7):1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Leigh SJ, Morris MJ. Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol basis Dis 1866;2020(6):165767. [DOI] [PubMed] [Google Scholar]
- [97].Rutsch A, Kantsjö JB, Ronchi F. The gut-brain Axis: how microbiota and host Inflammasome influence brain physiology and pathology. Front Immunol 2020; 11:604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36(5):305–12. [DOI] [PubMed] [Google Scholar]
- [99].Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 2015;172(11):1075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Koszewicz M, Jaroch J, Brzecka A, Ejma M, Budrewicz S, Mikhaleva LM, et al. Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment. Pharmacol Res 2021;164:105277. [DOI] [PubMed] [Google Scholar]
- [101].Torrallardona D, Harris CI, Fuller MF. Pigs’ gastrointestinal microflora provide them with essential amino acids. J Nutr 2003;133(4):1127–31. [DOI] [PubMed] [Google Scholar]
- [102].Mancin L, Wu GD, Paoli A. Gut microbiota-bile acid-skeletal muscle axis. Trends Microbiol 2023;31(3):254–69. [DOI] [PubMed] [Google Scholar]
- [103].Yadav A, Dahuja A, Dabur R. Dynamics of toll-like receptors signaling in skeletal muscle atrophy. Curr Med Chem 2021;28(28):5831–46. [DOI] [PubMed] [Google Scholar]
- [104].Yiu JH, Dorweiler B, Woo CW. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J Mol Med (Berl) 2017;95(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022;71(3):544–52. [DOI] [PubMed] [Google Scholar]
- [106].Chen Y, Gu S, Chen Y, Lu H, Shi D, Guo J, et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut 2022;71(1):222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70(4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tian Y, Sun KY, Meng TQ, Ye Z, Guo SM, Li ZM, et al. Gut microbiota may not be fully restored in recovered COVID-19 patients after 3-month recovery. Front Nutr 2021;8:638825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1(1): 56–66. [DOI] [PubMed] [Google Scholar]
- [110].Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022;11(1):e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Farhadfar N, Gharaibeh RZ, Dahl WJ, Mead L, Alabasi KM, Newsome R, et al. Gut microbiota dysbiosis associated with persistent fatigue in hematopoietic cell transplantation survivors. Transplant Cell Ther 2021;27(6). 498.e1–.e8. [DOI] [PubMed] [Google Scholar]
- [112].McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500(7464): 585–8. [DOI] [PubMed] [Google Scholar]
- [114].Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021;184(16). 4137–53.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. Jama 2021;326(11):1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cheema HA, Shahid A, Ayyan M, Mustafa B, Zahid A, Fatima M, et al. Probiotics for the prevention of ventilator-associated pneumonia: An updated systematic review and meta-analysis of randomised controlled trials. Nutrients 2022:14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wastyk HC, Perelman D, Topf M, Fragiadakis GK, Robinson JL, Sonnenburg JL, et al. Randomized controlled trial demonstrates response to a probiotic intervention for metabolic syndrome that may correspond to diet. Gut Microbes 2023;15(1):2178794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018;174(6). 1406–23.e16. [DOI] [PubMed] [Google Scholar]
- [119].Éliás AJ, Barna V, Patoni C, Demeter D, Veres DS, Bunduc S, et al. Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: a systematic review and meta-analysis. BMC Med 2023;21 (1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Leeuwendaal NK, Stanton C, O’Toole PW, Beresford TP. Fermented foods, health and the gut microbiome. Nutrients 2022:14(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Merenstein D, Fraser CM, Roberts RF, Liu T, Grant-Beurmann S, Tan TP, et al. Bifidobacterium animalis subsp. lactis BB-12 protects against antibiotic-induced functional and compositional changes in human fecal microbiome. Nutrients 2021:13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Varsha KK, Narisetty V, Brar KK, Madhavan A, Alphy MP, Sindhu R, et al. Bioactive metabolites in functional and fermented foods and their role as immunity booster and anti-viral innate mechanisms. J Food Sci Technol 2022;60 (9):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Muhialdin BJ, Zawawi N, Abdull Razis AF, Bakar J, Zarei M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control 2021;127:108140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Peters A, Krumbholz P, Jäger E, Heintz-Buschart A, Çakir MV, Rothemund S, et al. Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet 2019;15(5): e1008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, et al. Changes of the human gut microbiome induced by a fermented milk product. Sci Rep 2014;4: 6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yılmaz İ, Dolar ME, Özpınar H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turk J Gastroenterol 2019;30(3):242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yamamoto B, Suzuki Y, Yonezu T, Mizushima N, Watanabe N, Sato T, et al. Cha-Koji, comprising green tea leaves fermented with aspergillus luchuensis var kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci Biotechnol Biochem 2018;82(5):885–92. [DOI] [PubMed] [Google Scholar]
- [128].Inoguchi S, Ohashi Y, Narai-Kanayama A, Aso K, Nakagaki T, Fujisawa T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int J Food Sci Nutr 2012;63(4):402–10. [DOI] [PubMed] [Google Scholar]
- [129].Chiu HF, Chen YJ, Lu YY, Han YC, Shen YC, Venkatakrishnan K, et al. Regulatory efficacy of fermented plant extract on the intestinal microflora and lipid profile in mildly hypercholesterolemic individuals. J Food Drug Anal 2017;25(4):819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nielsen ES, Garnås E, Jensen KJ, Hansen LH, Olsen PS, Ritz C, et al. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation - a pilot study. Food Funct 2018;9(10):5323–35. [DOI] [PubMed] [Google Scholar]
- [131].Han K, Bose S, Wang JH, Kim BS, Kim MJ, Kim EJ, et al. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol Nutr Food Res 2015;59(5):1004–8. [DOI] [PubMed] [Google Scholar]
- [132].Taylor BC, Lejzerowicz F, Poirel M, Shaffer JP, Jiang L, Aksenov A, et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems 2020;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144(7). 1394–401, 401.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Cheon MJ, Lim SM, Lee NK, Paik HD. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int J Mol Sci 2020;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Murakami S, Miyazaki I, Asanuma M. Neuroprotective effect of fermented papaya preparation by activation of Nrf2 pathway in astrocytes. Nutr Neurosci 2018;21 (3):176–84. [DOI] [PubMed] [Google Scholar]
- [136].Ross RP, Morgan S, Hill C. Preservation and fermentation: past, present and future. Int J Food Microbiol 2002;79(1–2):3–16. [DOI] [PubMed] [Google Scholar]