Transforming activity of Fbxo7 is mediated specifically through regulation of cyclin D/cdk6 (original) (raw)
Abstract
D cyclins (D1, D2 and D3) and their catalytic subunits (cyclin-dependent kinases cdk4 and cdk6) have a facilitating, but nonessential, role in cell cycle entry. Tissue-specific functions for D-type cyclins and cdks have been reported; however, the biochemical properties of these kinases are indistinguishable. We report that an F box protein, Fbxo7, interacted with cellular and viral D cyclins and distinguished among the cdks that bind D-type cyclins, specifically binding cdk6, in vitro and in vivo. Fbxo7 specifically regulated D cyclin/cdk6 complexes: Fbxo7 knockdown decreased cdk6 association with cyclin and its overexpression increased D cyclin/cdk6 activity and E2F activity. Fbxo7 interacted with p27, but its enhancement of cyclin D/cdk6 activity was p21/p27 independent. Fbxo7 overexpression transformed murine fibroblasts, rendering them tumorigenic in athymic nude mice. Transformed phenotypes were dependent on cdk6, as knockdown of cdk6 reversed them. Fbxo7 was highly expressed in epithelial tumors, but not in normal tissues, suggesting that it may have a proto-oncogenic role in human cancers.
Keywords: cell cycle, cdk6, D cyclin, Fbxo7, transformation
Introduction
D cyclins (D1, D2, D3) are activators of cyclin-dependent kinases cdk4 and cdk6, whose activity ‘translates' growth signals into cell cycle progression (Morgan, 1995; Sherr, 1996). Mitogen-sensitive stages in D cyclin/cdk function include the transcription of cyclin genes and complex formation. Studies in mice showed that cyclin D null cells can proliferate normally in culture, demonstrating that D cyclins are not essential for the cell cycle. However, the ability of these cells to exit quiescence was impaired under low serum conditions, supporting the role of D cyclins as effectors of mitogenic signaling (Kozar et al, 2004).
The regulation of D cyclin/cdk complexes in vivo is complex and involves proteins that localize, fold, activate, export and degrade the subunits. Among the facilitators of D cyclin/cdk activity in vivo are SEI1 (Sugimoto et al, 1999, 2002), chaperone proteins, Hsc70 (Diehl et al, 2003), Hsp90 and Cdc37 (Dai et al, 1996; Stepanova et al, 1996) and cdk ‘inhibitors', p21 and p27. Cells lacking p21/p27 have a substantial reduction in D cyclin/cdk activity, and this is due in part to their facilitation of the assembly and import of these complexes into the nucleus (Diehl and Sherr, 1997; LaBaer et al, 1997; Cheng et al, 1999; Sherr and Roberts, 1999; Yang and Kornbluth, 1999; Alt et al, 2002; Sugimoto et al, 2002). Whether free or bound to cdk, the half-life of cyclin D1 is less than 30 min when cytoplasmic, compared to over 3 h in the nucleus. Instability is thought to be due to ubiquitin-mediated degradation (Diehl et al, 1997; Germain et al, 2000). Cyclin D1 phosphorylation by GSK-3β enables its Crm1-mediated export from the nucleus (Diehl et al, 1998; Alt et al, 2000). Export can be antagonized by p21, and sustained nuclear localization of cyclin D/cdk4 in NIH3T3 cells renders them tumorigenic (Alt et al, 2000, 2002). D cyclin/cdk complexes also titrate p21 and p27 away from cyclin/cdk2 complexes, facilitating their activation and cellular commitment to DNA synthesis (Parry et al, 1999; McConnell et al, 1999). Only a small number of substrates for D cyclin/cdk complexes have been identified, including the retinoblastoma family members (pRb, p107 and p130), which have an anti-proliferative role through repression of E2F transcription factors. Recently, it has been shown that a second anti-proliferative signaling pathway (TGF-β) is inactivated by cyclin D/cdk4 complexes through the phosphorylation of Smad3 (Matsuura et al, 2004).
The repertoire of proteins that facilitate entry into the cell cycle includes three D cyclins and two cdks. While it appears that there is some tissue specificity for cyclins expression and the use of a particular cdk, there is a high level of redundancy. Most cells express cdk4 and cdk6, although the activity of one seems to predominate. For example, D cyclin/cdk4 complexes are reported to be the main kinase in fibroblasts and macrophages, whereas D cyclin/cdk6 activity predominates in lymphocytes (Matsushime et al, 1994; Mahony et al, 1998; Parry et al, 1999). There are few functional distinctions between D cyclin/cdk4 and D cyclin/cdk6 complexes; however, the ability to alter astrocytes morphology and to prevent terminal differentiation of erythrocytes is attributed specifically to cdk6 (Ericson et al, 2003; Matushansky et al, 2003).
D cyclins are frequently mutated in cancers by retroviral integration, gene amplification or chromosomal translocation (Bates and Peters, 1995; Hall and Peters, 1996; Sherr, 1996), and upregulated in many cancers, including head and neck cancers, B-cell lymphomas, parathyroid adenomas and breast cancers (Sherr, 1995, 1996). Expression of cdk4 or cdk6 is also increased in malignancies such as B-cell lymphomas and glioblastomas (Khatib et al, 1993; Schmidt et al, 1994; Chilosi et al, 1998; Corcoran et al, 1999; Hayette et al, 2003). Notably, cells lacking D cyclins or cdk4/cdk6 exhibit resistance to transformation by known oncogenes, emphasizing the importance of this pathway in tumorigenesis (Zou et al, 2002; Kozar et al, 2004; Malumbres et al, 2004).
This study describes a novel interaction for Fbxo7, an F box domain protein. Fbxo7 was cloned in a yeast two-hybrid screen as interacting with a viral cyclin. F box proteins are specificity determining subunits for Skp1/cullin/F box protein (SCF) E3 ubiquitin ligases (Deshaies, 1999; Jackson and Eldridge, 2002). As some of their targets impact the cell cycle, they are also thought to be involved in oncogenesis (Bashir and Pagano, 2003; Pagano and Benmaamar, 2003). Although these ligases have mostly been described as precipitating the ubiquitin-mediated degradation of their substrates, it is being increasingly appreciated that ubiquitination is a multifunctional modification, affecting subcellular localization, receptor endocytosis, protein sorting and trafficking (Haglund et al, 2003; Hicke and Dunn, 2003; Horak, 2003; Shcherbik and Haines, 2004). Fbxo7 was required specifically for D cyclin/cdk6 activity. Fbxo7 expression in NIH3T3 cells increased the levels of cyclin D/cdk6 complexes, elevated E2F activity and caused transformation. Moreover, transformed phenotypes required cdk6, as its knockdown reversed these characteristics. Fbxo7 was expressed in lung and colon cancers but not in the corresponding normal tissues, suggesting that it is an oncoprotein in human malignancies.
Results
Fbxo7 interacted with a viral cyclin
The cyclins encoded by oncogenic herpesviruses are homologous to D cyclins. Uniquely, viral cyclins also phosphorylate cyclin/cdk2 substrates and do not bind the cdki or INK4a family proteins (reviewed in Laman et al, 2000). To identify proteins important in viral or D cyclin-mediated oncogenesis, a yeast two-hybrid screen of a B- and T-cell cDNA library was undertaken using the Herpesvirus saimiri (HVS) cyclin as bait (Laman et al, 2001). A single clone encoding aa 129–393 of Fbxo7 fused with Gal4 activation domain (GAD) was isolated (Figure 1A). The specificity of Fbxo7 interaction with HVS cyclin was tested by assaying for activation of the HIS3 and ADE2 reporter genes. Yeast cotransformed with pGBD-HVS cyclin and pGAD-Fbxo7 grew on media lacking histidine and adenine. Yeast co-transformed with either pGBD or pGBD-cyclin E and pGAD-Fbxo7 as well as yeast co-transformed with pGBD-HVS cyclin and pGAD-SV40 large T antigen were unable to grow on media lacking histidine and adenine, indicating no activation of the reporter genes (Figure 1B). These data argue for the specificity of the interaction of viral cyclin with Fbxo7.
Figure 1.
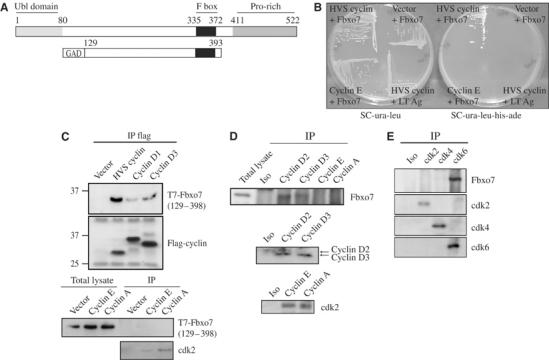
(A) Schematic diagram of Fbxo7, with F box motif (black), proline-rich domain (medium gray) and Ubl motif (light gray). Sequences contained within pGAD-Fbxo7 are indicated. (B) pGAD-Fbxo7 interacted specifically with pGBD-HVS cyclin. Yeast were grown on media selecting for the plasmids (SC-ura-leu) and on media selecting for activation of reporter genes (SC-ura-leu-his-ade). (C) Western blot of T7-Fbxo7(129–398) detected in anti-flag immunoprecipitates of D cyclins, but not cyclins E and A (bottom panels). Western blot of cdk2 is shown as a control for equivalent immunoprecipitation of these cyclins. (D) Fbxo7 co-immunoprecipitated with D cyclins, but not cyclins E or A. Western blots of cyclins D2 and D3 (middle), and cdk2 (bottom) shown as controls for cyclin immunoprecipitation. (E) Fbxo7 was detected in immunoprecipitates of cdk6, but not cdk4 or cdk2. Western blots for cdk subunits are shown below.
Fbxo7 interacted with viral and cellular D cyclins and specifically with cdk6
To extend this finding to mammalian cells and to cellular cyclins, U2OS cells were co-transfected with a T7-Fbxo7(129–398) clone and flag-HVS cyclin, cyclin D1 or D3. Both D cyclins and HVS cyclin co-immunoprecipitated with Fbxo7(129–398) (Figure 1C). Immunoprecipitations of cyclins E and A were also tested. Although cdk2 interacted with both cyclins, Fbxo7 did not. These data demonstrated that Fbxo7 interacted with D cyclins in human cells. In addition, these data indicated that aa 1–128 and 399–522 of Fbxo7 were dispensable for in vivo interaction.
This interaction was further investigated by immunoprecipitating endogenous cyclins from U2OS cell lysates and assaying for endogenous Fbxo7 using an antibody against its C-terminus. In co-immunoprecipitation experiments, D cyclins interacted with Fbxo7, while cyclins E and A interacted with cdk2, but not Fbxo7 (Figure 1D). Thus, Fbxo7 interacted in vivo specifically with D cyclins at their endogenous levels of expression.
D cyclins activate several cdks (2, 4 and 6) with which Fbxo7 might also interact. To test whether Fbxo7 interacted with a particular cdk, immunoprecipitations were performed with antibodies to endogenous cdks. Western blots for Fbxo7 showed specific co-immunoprecipitation of Fbxo7 with cdk6, but not with cdk2 or cdk4 (Figure 1E). Thus, Fbxo7 distinguished among the cdks to interact specifically with cdk6.
To determine whether the cyclin, cdk or both subunits interacted directly with Fbxo7, in vitro binding assays were performed. GST-D cyclins or GST-cdks were bacterially expressed and purified (Figure 2A), and equivalent amounts of GST proteins were used in binding assays where Fbxo7 was in vitro transcribed and translated in reticulocyte lysates. Fbxo7 showed a strong interaction with GST-cdk6 fusion protein, but not with GST alone or GST fusions to cyclin D1, cyclin D3 or cdk4 (Figure 2B), indicating that Fbxo7 bound directly to cdk6 in vitro.
Figure 2.
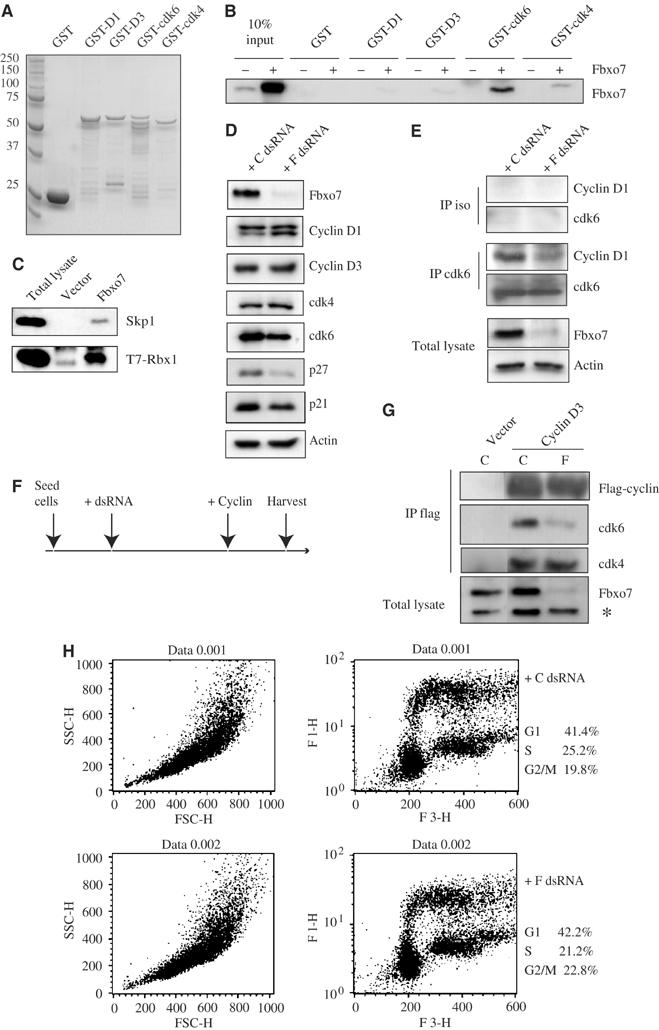
(A) Coomassie staining of an SDS–PAGE gel showing GST fusion proteins to cyclin and cdk subunits. (B) GST-cdk6, but not GST, GST-cyclin D1/D3 or GST-cdk4, bound _in vitro_-translated Fbxo7 protein. (C) Western blots for Skp1 and T7 of anti-flag immunoprecipitates of lysates of U2OS cells cotransfected with flag-Fbxo7 and T7-Rbx1. (D) Western blots for cell cycle proteins of lysates from cells transfected with control (C) or Fbxo7 (F) dsRNA. (E) Western blots of anti-cdk6 or isotype control (iso) immunoprecipitates for cdk6 and cyclin D1 from cells with endogenous or reduced Fbxo7. (F) Timeline of de novo association experiments conducted in panel G and Figure 3E. (G) Western blots for cdks and flag of anti-flag immunoprecipitates from cells with endogenous or reduced Fbxo7. Western blots of total lysates for Fbxo7 are shown. * indicates a nonspecific band used as an internal loading control. (H) FACS analysis of BrdU incorporation by control (C) or Fbxo7 (F) dsRNA-treated cells stained with FITC-conjugated anti-BrdU antibodies (FL1-H) and propidium iodide (FL3-H).
Fbxo7 facilitated cyclin D association with cdk6 specifically
Fbxo7 has previously been identified in screens for proteins with F box domains, which bind Skp1 (Cenciarelli et al, 1999; Winston et al, 1999; Ilyin et al, 2000). The presence of this motif suggests that this protein is a component of an SCF-E3 ubiquitin ligase. We also tested whether Fbxo7 interacted with components of these ligases. A construct encoding flag-Fbxo7 was co-transfected with T7-Rbx1 into U2OS cells. Among the proteins detected in flag immunoprecipitates of the cell lysates were T7-Rbx1 and endogenous Skp1 (Figure 2C), suggesting that Fbxo7 formed part of an SCF-E3 ubiquitin ligase in vivo.
In addition to the F box motif and proline-rich domains, analysis of the primary sequence also revealed an N-terminal ubiquitin-like (Ubl) domain spanning aa 1–80 (Figure 1A). Database searches for human Fbxo7 homologs identified other F box proteins, Fbxo9 and Fbxo11, with homology to Fbxo7, but only within the F box domain (data not shown). Fbxo7 homologs were identified in other vertebrate organisms (Supplementary Figure 1). In addition, Arabidopsis thaliana has two and Oryza sativa has three partial homologs to Fbxo7, which lack Ubl domains (data not shown). However, none were found in Caenorhabditis elegans, Drosophila melanogaster or Saccharomyces cerevisiae.
The interaction of Fbxo7 with components of E3 ubiquitin ligases as well as D cyclins and cdk6 suggested that Fbxo7 may ubiquitinate these proteins. One consequence of this modification is ubiquitin-mediated degradation by the 26S proteasome. If this were the case, then reducing Fbxo7 levels should increase the abundance of D cyclins or cdk6. To investigate this, Fbxo7 levels were reduced by transfecting double-stranded RNA (dsRNA) targeting the mRNA. Western blots of lysate from cells transfected with dsRNA against Fbxo7 showed that Fbxo7 levels were reduced >90% as compared to lysates transfected with a nonsilencing control (Figure 2D). The correlative effects on other cell cycle regulators were also investigated. There was a small decrease in the amount of cdk6; however, cdk4 and D cyclin levels were unchanged. In addition, the levels of p21 and p27 proteins, which interact with cyclin D/cdk complexes, were reduced (Figure 2D). As a decrease in Fbxo7 levels did not correlate with increased levels of D cyclins or cdk6, these data argue against a role for Fbxo7 in facilitating their ubiquitin-mediated degradation. Instead, Fbxo7 knockdown correlated with a reduction in assembly factors for D cyclin/cdk complexes, suggesting that Fbxo7 might positively regulate them.
To begin to address this, the abundance of D cyclin/cdk6 complexes when Fbxo7 protein levels were reduced was tested. dsRNA was transfected into U2OS cells as above, lysates were immunoprecipitated with antibodies to cdk6, and the amount of cyclin D1 was assayed by Western blotting. In immunoprecipitates of equivalent amounts of cdk6, cyclin D1 levels were substantially reduced when Fbxo7 protein was decreased (Figure 2E). Thus, levels of endogenous cyclin D1/cdk6 complexes correlated with Fbxo7 abundance.
We assessed the ability of D cyclins to assemble de novo with cdk6, when Fbxo7 was reduced. A schematic of these experiments is outlined in Figure 2F. After Fbxo7 knockdown, flag-cyclin D3 was introduced. Flag immunoprecipitates from these cell lysates were assayed for associated cdks. Cyclin D3 co-immunoprecipitated cdk4 and cdk6. However, when Fbxo7 levels were reduced, there was a marked and specific reduction in the levels of associated cdk6. The amount of cdk4 associated with cyclin D3 was not affected (Figure 2G). The effect of decreasing Fbxo7 levels on cell cycle was assayed by measuring BrdU incorporation. No change of cell cycle parameters was seen in cells with reduced Fbxo7 compared to endogenous levels (Figure 2H). Thus, decreased D cyclin/cdk6 complexes were not a consequence of cell cycle perturbation resulting from reduced Fbxo7, and also decreasing Fbxo7 did not alter cell cycle progression.
Knockdown of Fbxo7 correlated with reduced levels of p27, and to a lesser extent p21. These proteins potentiate cyclin D/cdk complexes by several mechanisms: facilitating their stabilization, nuclear import and nuclear retention of cyclin D/cdk complexes (LaBaer et al, 1997; Cheng et al, 1999; Alt et al, 2002). To determine if Fbxo7 also interacted with p21 and p27, cells were transfected with Fbxo7 and lysates were immunoprecipitated with antibodies to p21 and p27 and assayed for Fbxo7. Fbxo7 was detected in immunoprecipitates of p27, but not p21 (Figure 3A), demonstrating that Fbxo7 interacted in vivo with this positive regulator for cyclin D/cdk complexes.
Figure 3.
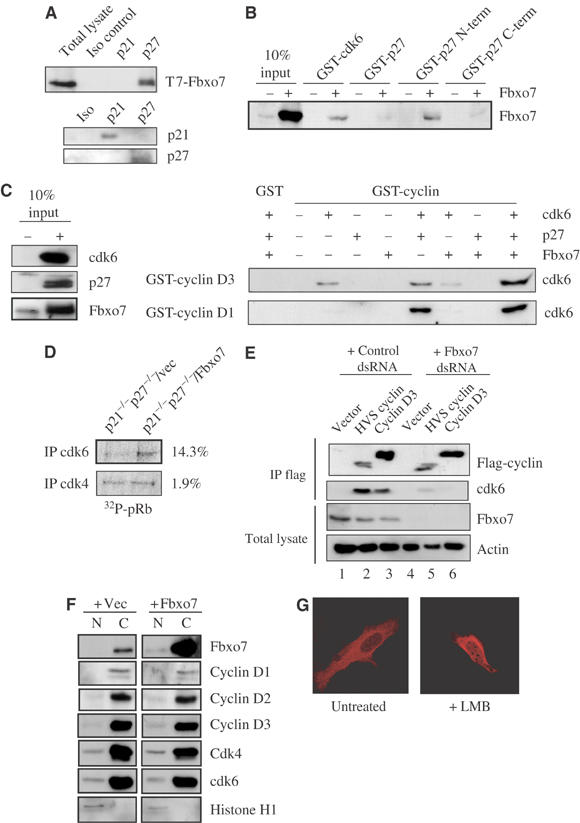
(A) Western blots for T7-Fbxo7, p21 and p27 in anti-p21 and p27 immunoprecipitates from lysates of T7-Fbxo7-transfected cells. (B) GST-cdk6 and GST-p27(N-term) interacted with _in vitro_-transcribed and -translated Fbxo7. (C) Western blot for cdk6 in binding assays using GST-cyclin D1 and GST-cyclin D3 and _in vitro_-translated proteins, cdk6, p27 and Fbxo7. Western blot of 10% input is shown in the left panel. (D) A representative result of an in vitro kinase assay of anti-cdk immunoprecipitates from p21−/−p27−/−cells, with or without Fbxo7, using pRb as a substrate. The average percent increase from three experiments is noted. (E) Western blots for cdk6 and flag of anti-flag immunoprecipitates from cells with endogenous or reduced Fbxo7 and of total lysates for Fbxo7. Experiment was performed as in Figure 2G. (F) Western blots of nuclear or cytoplasmic fractions from cells with endogenous or overexpressed Fbxo7. (G) Images of confocal fluorescence microscopy of untreated or leptomycin B (LMB)-treated cells expressing dsRED-Fbxo7.
To determine whether p27 interacted directly with Fbxo7, in vitro binding assays were performed in an identical manner as in Figure 2B. GST fusion proteins to p27, p27 N-terminus (aa 1–91) and p27 C-terminus (aa 107–198) were used in binding assays with _in vitro_-translated Fbxo7. Fbxo7 bound GST-p27 N-terminus with an efficiency equivalent to its binding to GST-cdk6 (Figure 3B). Together, these data provide evidence that Fbxo7 interacted with D cyclins, cdk6 and p27 in vivo and with GST-cdk6 and GST-p27 N-terminus in binding assays.
Fbxo7 with p27 facilitated cyclin D3/cdk6 binding in vitro
Fbxo7 interacted with proteins, which together form an active complex in vivo, suggesting that Fbxo7 might function to assemble a ternary complex. To test this hypothesis directly, in vitro binding assays were performed using p27 and Fbxo7 to promote the interaction of cdk6 with GST-D cyclins. As seen in Figure 3C, GST-cyclin D1 did not bind to cdk6 alone, but p27 greatly increased their association. The presence of Fbxo7 alone or in combination with p27 did not further enhance GST-cyclin D1 binding to cdk6, arguing against Fbxo7 functioning as an assembly factor for cyclin D1/cdk6 complexes. In contrast to GST-cyclin D1, GST-cyclin D3 interacted directly with cdk6 in the absence of p27 and Fbxo7. The addition of neither protein increased its binding. In fact, Fbxo7 slightly inhibited GST-cyclin D3 binding to cdk6. However, when cdk6, p27 and Fbxo7 were present together, there was a synergistic increase in the amount of cdk6 bound by GST-cyclin D3. These data argue that D cyclins differ in their capacity to associate with cdk6, and furthermore that cyclin D3, but not cyclin D1, is sensitive to the presence of Fbxo7 for in vitro binding to cdk6.
p21/p27 were not required for Fbxo7 function in vivo
Binding assays indicated Fbxo7 required p27 to stimulate cdk6 association with cyclin D3, while p27 was essential for cdk6 binding to cyclin D1. Although p21 and p27 potentiate D-cyclin/cdk complexes both in vivo and in vitro, their activity is not essential for cyclin D/cdk activity in vivo, suggesting that other cofactors function in their absence (Cheng et al, 1999; Sugimoto et al, 2002; Bagui et al, 2003). To test whether Fbxo7 required p21/p27 to enhance cdk6 activity in vivo, p21−/−p27−/− MEFs were infected with retroviruses encoding either Fbxo7 or empty vector. In vitro kinase assays were performed on anti-cdk immunoprecipitates using pRb as a substrate. It has been previously reported that p21−/−p27−/− cells have substantially reduced cyclin D/cdk activity (Sugimoto et al, 2002; Bagui et al, 2003), which we also observed (data not shown). However, p21−/−p27−/−/Fbxo7 MEFs had an ∼15% increase in cdk6 activity over p21−/−p27−/−/vec MEFs, although they had similar levels of cdk4 activity (Figure 3D). These data indicated that Fbxo7 increased cdk6 activity specifically in the absence of p21/p27.
The viral cyclins preferentially form complexes with cdk6, and do not bind p21 or p27 (Swanton et al, 1997). To determine whether viral cyclin association with cdk6 was dependent on Fbxo7, HVS cyclin was tested for its de novo association with cdk6 in an identical manner as in Figure 2G. HVS cyclin co-immunoprecipitated cdk6; however, the amount was substantially reduced when Fbxo7 was decreased (Figure 3E), indicating that Fbxo7 enhanced viral cyclin association with cdk6. These data suggest that Fbxo7 had a facilitating role in cyclin/cdk6 association, which is independent of p21/p27 activity in vivo.
Fbxo7 is exported from the nucleus and located predominantly in the cytoplasm
Individual cyclin and cdk subunits are present in excess in the cytoplasm, where complex assembly is thought to occur. As the data suggest that Fbxo7 can promote cyclin/cdk association, the subcellular localization of Fbxo7 was investigated. Asynchronous U2OS cells were fractionated into nuclear and cytoplasmic compartments, and the extracts were assayed by Western blotting for D cyclins, cdks and Fbxo7. The predominant localization of endogenous Fbxo7, D cyclin and cdk subunits was in the cytoplasm (Figure 3F). In addition, when Fbxo7 was overexpressed by transient transfection, the exogenous protein was present in the cytoplasm, with small amounts being detected in the nucleus. Notably, the subcellular localization of the D cyclins and cdk subunits was not dramatically altered when Fbxo7 was overexpressed (Figure 3F). As these data suggested that Fbxo7 could be localized to the nucleus, a fusion of dsRED to Fbxo7 was created and transfected into U2OS cells. When asynchronous cells were visualized by confocal fluorescence microscopy, dsRED-Fbxo7 was localized exclusively to the cytoplasm in all cells observed. However, when cells were treated with leptomycin B, an inhibitor of nuclear export, dsRED-Fbxo7 was detected in the nucleus (Figure 3G). This suggested that nuclear Fbxo7 was actively exported by a Crm1-dependent mechanism.
Fbxo7 expression in murine fibroblasts enhanced cyclin D/cdk6 activity
Since Fbxo7 specifically affected cyclin D/cdk6 complexes, the effect of its overexpression on cyclin D/cdk6 activity and its activity in a transformation model were tested. NIH3T3 cells were engineered to stably overexpress T7-Fbxo7. Clonal and polyclonal 3T3/Fbxo7 and 3T3/vector cell lines were created, and both polyclonal and clonal cell lines behaved similarly in all assays. Fbxo7 expression was tested by Western blotting for T7. Cdk4 and cdk6 levels were also tested and found not to be significantly altered (Figure 4A). Endogenous cdk activity was next investigated. Cdk4 and cdk6 were immunoprecipitated from lysates of 3T3/vec or 3T3/Fbxo7 cells and tested in in vitro kinase assays against a pRb substrate. Quantification of 32P incorporation showed that cdk6 activity was 36.3% higher in 3T3/Fbxo7 cells compared to the control, while the amount of cdk4 activity was 2.2% greater (Figure 4B). Increased cdk6 activity correlated with increased E2F activity, as determined by E2F-luciferase reporter assays. 3T3/Fbxo7 cells had an average of 2.3-fold higher activity than 3T3/vec cells (data not shown). Gene expression microarray analysis showed that E2F-regulated genes, including cyclin E, E2F1, mcm7, thymidylate synthase, ribonucleotide reductase M2, were significantly upregulated in 3T3/Fbxo7 cells (Figure 4C). Levels of D cyclin/cdk4 and D cyclin/cdk6 complexes were also assayed. 3T3/vec or 3T3/Fbxo7 cells were cotransfected with either flag-cyclin D1 or D3 with cdk4 or cdk6. In 3T3/vec cells, cyclin D1 associated almost exclusively with cdk4, while cyclin D3 bound cdk4 and cdk6 (Figure 4D). In 3T3/Fbxo7 cells, greater amounts of cdk6 associated with both cyclin D1 and cyclin D3, while the levels of associated cdk4 remained unchanged. Thus, Fbxo7 expression specifically increased levels of D cyclin/cdk6 complexes, but no specificity for a particular D cyclin was observed. These data confirmed that Fbxo7 has a positive effect on cyclin D/cdk6 complex levels and activity.
Figure 4.
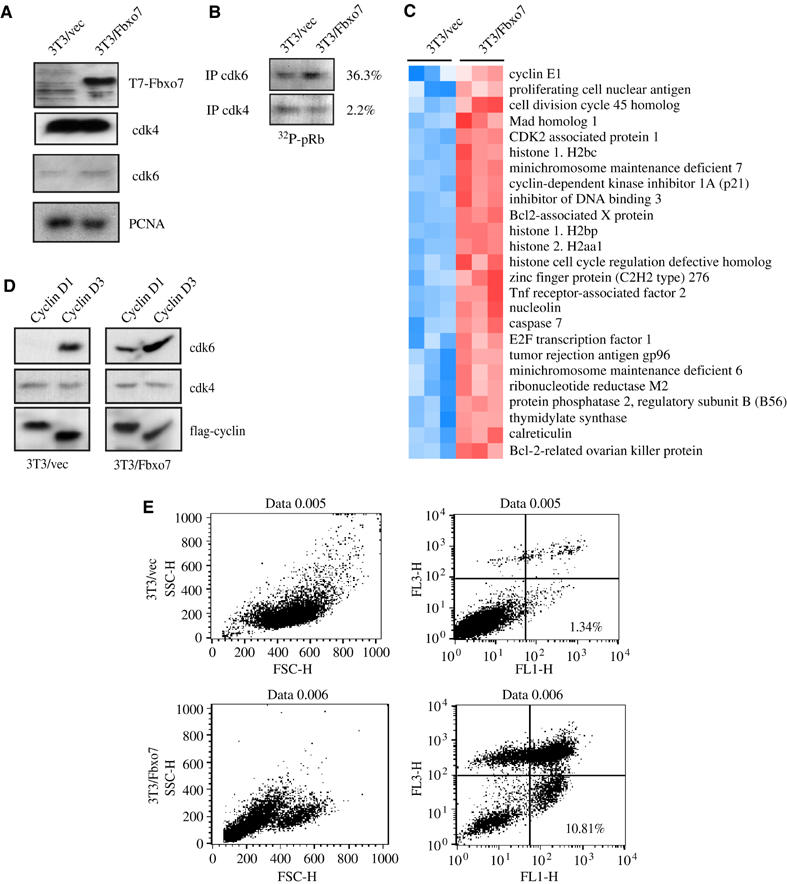
(A) Western analysis for T7-Fbxo7 and cdks in lysates from 3T3/vec or 3T3/Fbxo7 cells. (B) A representative result of an in vitro kinase assay of anti-cdk immunoprecipitates from 3T3/vec or 3T3/Fbxo7 cells using pRb as substrate. The average percent increase from three trials is noted. (C) Heat maps of E2F-regulated genes from GEM analysis of 3T3/vec and 3T3/Fbxo7 cells. Arrays were performed in triplicate. Red shows increased and blue decreased relative expression. (D) Western blots of flag and cdk subunits in anti-flag cyclin immunoprecipitates from 3T3/vec and 3T3/Fbxo7 cells. (E) FACS analysis on serum-starved 3T3/vec and 3T3Fbxo7 cells stained with FITC-conjugated antibodies to annexin V (FL1-H) and propidium iodide (FL3-H).
The data suggest that Fbxo7 cells have increased cyclin D/cdk6 activity, which is downstream of extracellular growth signaling. To determine whether increased Fbxo7 expression could substitute for the presence of external growth factors, 3T3/vec and 3T3/Fbxo7 cells were cultured in media with reduced serum (0.5%). In contrast to 3T3/vec cells, which withdrew from the cell cycle, as measured by BrdU incorporation (data not shown), 3T3/Fbxo7 cells became rounded and detached from the culture dish. To investigate if this was due to apoptosis, cells were stained with annexin V, an early marker for apoptosis, and propidium iodide. As seen in Figure 4E, in media with reduced serum, 10.81% of 3T3/Fbxo7 cells, compared to 1.34% of 3T3/vec cells, stained with annexin V but not propidium iodide, indicating that these cells were in the early stages of apoptosis. Overall, only 16.5% of 3T3/Fbxo7 cells were viable, compared to 88.2% of 3T3/vec cells after 72 h in reduced serum. Thus, Fbxo7 expression did not allow continued cell cycle progression, when growth factors were withdrawn, but rather resulted in apoptosis.
Fbxo7 expression transformed murine fibroblasts
We next investigated whether 3T3/Fbxo7 cells had transformed phenotypes. 3T3/vec or 3T3/Fbxo7 cells were tested for anchorage-independent growth by seeding into soft agar. Results from a representative experiment are shown in Figure 5A, where five-fold more colonies grew from 3T3/Fbxo7 cells compared to 3T3/vec cells. Fbxo7-expressing cells were also tested in a tumor formation assay. Two inocula of either 3T3/vec or 3T3/Fbxo7 cells were injected subcutaneously into the flanks of athymic nude mice. All eight sites inoculated with 3T3/Fbxo7 cells developed tumors, with a mean size of approximately 258 mm2 (Figure 5B). One of the eight sites inoculated with 3T3/vec cells developed a tumor, which did not grow >1 mm2. Tumors derived from 3T3/Fbxo7 cells were histologically fibrosarcomas, and analysis by immunostaining of tissues showed them staining strongly positive for Fbxo7 (Figure 5C).
Figure 5.
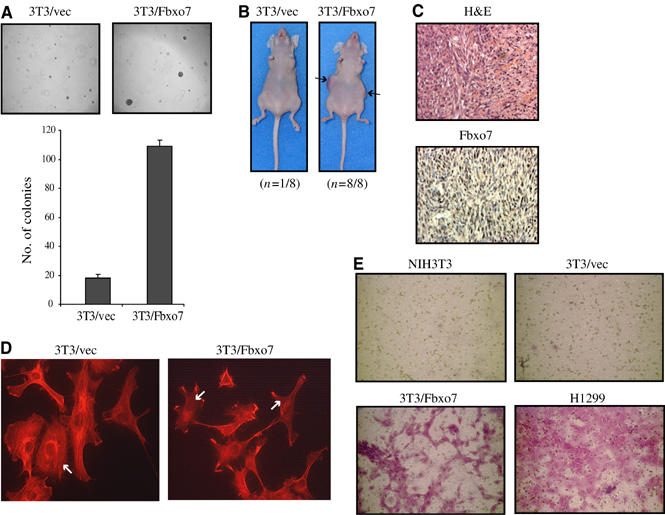
(A) Images of colony growth in soft agar assays (top) and graph of colony number formed by 3T3/vec and 3T3/Fbxo7 cells (bottom). (B) Tumor formation assay with 3T3/vec and 3T3/Fbxo7 cells. (C) Images of tumors from 3T3/Fbxo7 cells stained for histology with hematoxylin and eosin (H&E), and immunohistochemistry for Fbxo7. (D) Images from confocal fluorescence microscopy of cells stained with rhodamine-conjugated phalloidin. 3T3/Fbxo7 cells had disorganized F-actin and fewer stress fibers (indicated by white arrows). (E) Images of ECM invasion assays where invasive cells stain violet.
When visualized by light microscopy, 3T3/Fbxo7 cells had fewer lamellipodia and many filamentous projections (data not shown). This morphological change was investigated by observing the actin cytoskeleton using rhodamine conjugated-phalloidin. 3T3/Fbxo7 cells had fewer stress fibers and a disorganized actin cytoskeleton compared to 3T3/vec cells (Figure 5D). Because of these changes, the ability of cells to invade a reconstituted extracellular matrix (ECM) was tested. Invasive cells migrated through ECM and adhered to the underside of a supporting membrane, which was stained to visualize the cells. Whereas parental NIH3T3 cells and 3T3/vec cells were non-invasive, 3T3/Fbxo7 cells invaded the ECM. H1299 cells were used as a positive control (Figure 5E). Thus, Fbxo7 expression conferred transformed properties to NIH3T3 cells.
Requirements for Fbxo7-mediated transformation
Fbxo7 has motifs indicative of a role in the ubiquitin–proteasome pathway, which may be required for its transforming activity. In order to test this, a mutant truncating the N-terminal 129 aa, which includes the Ubl domain, was created, Fbxo7(129–522), and this construct was used to engineer an NIH3T3 cell line, 3T3/Fbxo7Δ129. Expression of Fbxo7(129–522) was confirmed by immunoblotting for Fbxo7 (Figure 6A). When compared to the vector cell line, 3T3/Fbxo7Δ129 cells invaded an ECM (Figure 6B) and grew as colonies in soft agar (Figure 6C), indicating that expression of Fbxo7Δ129 allowed anchorage-independent growth. These data indicate that the Ubl domain within the N-terminus was dispensable for the transforming activity of Fbxo7.
Figure 6.
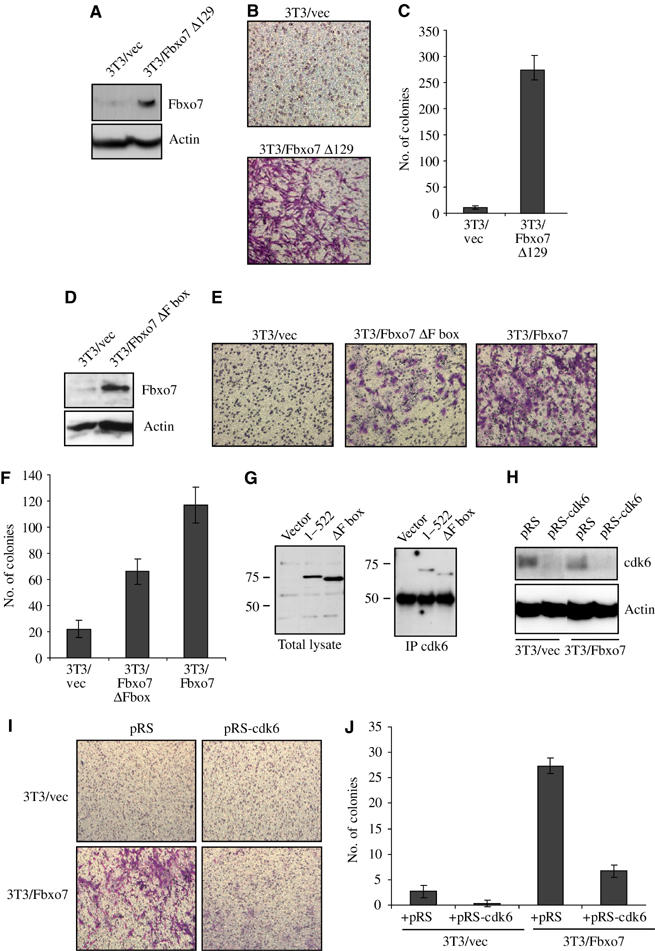
(A) Western blot for Fbxo7(129–522) expression in NIH3T3 cells. (B) Images from ECM invasion assays with 3T3/Fbxo7Δ129 cells. (C) Graph of colony number formed by 3T3/vec and 3T3/Fbxo7Δ129 cells in soft agar assay. (D) Western blot for Fbxo7ΔFbox expression in NIH3T3 cells. (E) Images from ECM invasion assays with 3T3/Fbxo7ΔFbox cells. (F) Graph of colony number for 3T3/Fbxo7ΔFbox cells in soft agar assay. (G) Western blot for the T7 from total lysate and anti-cdk6 immunoprecipitates from lysates of cells transfected with T7-Fbxo7 or T7-Fbxo7(ΔFbox). (H) Western analysis for cdk6 in cell lysates as indicated. (I) Images from ECM invasion assays with indicated cell lines. (J) Graph of number of colonies in soft agar assay with cell lines as indicated.
To test the requirement for the ubiquitin ligase function in transformation, a mutant of Fbxo7 deleting aa 335–367 (Fbxo7ΔFbox) was engineered. This mutant did not bind Skp1 in vivo (data not shown). An NIH3T3 cell line was created and expression of Fbxo7ΔFbox in cells was verified by immunoblotting for Fbxo7 (Figure 6D). Invasion assays indicated that the capacity of 3T3/Fbxo7ΔFbox cells to invade ECM was reduced relative to 3T3/Fbxo7 cells (Figure 6E). In addition, the ability of 3T3/Fbxo7ΔFbox cells to grow colonies in soft agar was also reduced 44% compared to wild-type Fbxo7 (_P_-value=0.0063) (Figure 6F). Thus, 3T3/Fbxo7ΔFbox cells had a less transformed phenotype, which indicated that the F box domain contributed significantly to the transforming capacity of Fbxo7.
While the presence of the F box domain augmented Fbxo7 transformation, 3T3/Fbxo7ΔFbox cells were also transformed. The previous data suggested that Fbxo7 transformed cells through its effects on cyclin D/cdk6 complexes. To test whether the F box was required for cdk6 interaction, U2OS cells were transfected with T7-Fbxo7 and T7-Fbxo7ΔFbox. Lysates from these cells were immunoprecipitated with antibodies to cdk6 and analyzed for Fbxo7. Both the full length and Fbxo7(ΔFbox) were present in immunoprecipitates of cdk6 (Figure 6G), indicating that the F box domain was dispensable for interaction with cdk6.
Knockdown of cdk6 reversed Fbxo7-mediated transformation
In order to test whether Fbxo7-mediated transformation was dependent on cdk6, siRNA was used to knock down cdk6 levels. A short hairpin construct with sequences targeting murine cdk6 was cloned into pRETROSUPER (pRS) to create pRS-cdk6. Retroviruses were used to infect 3T3/vec and 3T3/Fbxo7 cells and polyclonal cell lines were assayed for the expression of cdk6. Infection with pRS-cdk6 retroviruses caused a substantial reduction in cdk6 levels (Figure 6H). To test whether the invasive capacity of Fbxo7-expressing cells was dependent on cdk6, 3T3/vec/pRS, 3T3/vec/pRS-cdk6, 3T3/Fbxo7/pRS and 3T3/Fbxo7/pRS-cdk6 cells were seeded into ECM invasion chambers. Reduction of cdk6 levels ablated the invasiveness of 3T3/Fbxo7 cells (Figure 6I). In addition, when tested for anchorage-independent growth, fewer colonies grew when 3T3/Fbxo7/pRS-cdk6 cells were seeded in soft agar compared to 3T3/Fbxo7/pRS cells (Figure 6J). Neither 3T3/vec/pRS cells nor 3T3/vec/pRS-cdk6 cells formed colonies in soft agar. These data indicated that the transformed phenotypes resulting from the expression of Fbxo7 were dependent on cdk6, and implicated Fbxo7 and cdk6 as regulators of transformation and invasiveness.
Fbxo7 was expressed in tumors of the lung and colon
To begin to address a proto-oncogenic role for Fbxo7 in human cancers, its expression in epithelial tumors was assessed by comparing normal and cancerous sections from lung and colon on a multiple tumor tissue array. Fbxo7 was not detected in normal tissues of the colon (_n_=4) and lung (_n_=7), but was highly expressed in all cases of colorectal adenocarcinoma (_n_=40) and lung cancer (squamous cell carcinoma and adenocarcinoma; _n_=49) tested (Figure 7A and B). In addition, the subcellular localization of Fbxo7 was cytoplasmic in 10% of colon cancers and 65% of lung cancers, but both nuclear and cytoplasmic in 90% and 35% of these samples, respectively. These data demonstrate that Fbxo7 was expressed in tumors of epithelial lineages, but not in the corresponding normal tissues, and suggested that Fbxo7 expression and localization might be deregulated in tumors.
Figure 7.
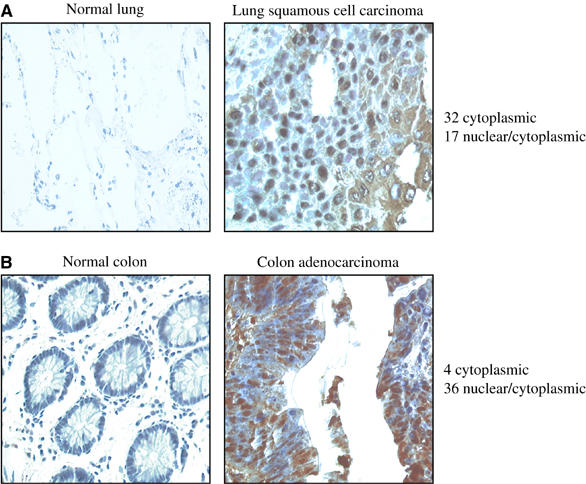
(A) Images of immunohistochemistry for Fbxo7 on normal lung tissue and biopsies from lung cancers. Brown color indicates Fbxo7 staining, and hematoxylin counterstaining for nuclei is blue. Scoring of subcellular localization of Fbxo7 is summarized. (B) Images of immunohistochemistry for Fbxo7 in normal colon and in malignancies of the colon. Staining and analysis are as above.
Discussion
The data presented here provide evidence that Fbxo7, an F box domain containing protein, interacts with and positively regulates cell cycle proteins. Several lines of evidence support the interaction of Fbxo7 with D cyclins, cdk6 and p27, including yeast two-hybrid data, in vivo co-immunoprecipitation assays and in vitro binding assays. This interaction was discovered by virtue of an interaction with a viral cyclin in a yeast two-hybrid screen (Figure 1B). In binding assays, Fbxo7 interacted directly with cdk6, but not with cyclins (Figure 2B), suggesting that for Fbxo7 to be cloned in the yeast two-hybrid screen, the viral cyclin recruited endogenous Cdc28 kinase, which bridged the interaction. Other proteins cloned in this screen include the cyclin/cdk assembly factor, Sei1 (our unpublished results) and cdk substrates, like Orc1 (Laman et al, 2001), also suggesting that viral cyclin/Cdc28 complexes might mediate these interactions.
Although cloned by HVS cyclin, Fbxo7 also interacted with cellular D cyclins, and specifically with cdk6. The specificity of Fbxo7 for cdk6 is surprising, as cdk4 and cdk6 are homologous. In addition, Fbxo7 also interacted in vivo and in vitro with p27. Fbxo7 interaction with three components of active kinases suggested that it might act as an assembly factor. Fbxo7 did not facilitate cyclin D1/cdk6 binding nor did Fbxo7 alone facilitate cyclin D3/cdk6 binding in vitro; however, in the presence of p27 and Fbxo7, cyclin D3 bound increased amounts of cdk6, while cyclin D1 was unaffected (Figure 3C). Possible mechanisms for Fbxo7 are that it acts as a scaffold or chaperone to fold or stabilize cyclin D3/cdk6/p27 complexes, at this stage of cdk6 activation. Interestingly, cyclin D1 and cyclin D3 differed in cdk6 binding, suggesting that their assembly is based on different affinities for the cdks and the availability of and sensitivity to different cofactors.
Although p27 was necessary for Fbxo7 to increase cyclin D3/cdk6 association in vitro (Figure 3C), Fbxo7 increased cdk6 activity in the absence of p21/p27 in vivo (Figure 3D). The presence of other proteins (like p57, Hsc70, Cdc37) may substitute for p21/p27 assembly in vivo or may indicate that Fbxo7 has additional mechanisms to increase cdk6 activity other than promoting complex assembly. For example, cyclin D1/cdk6 binding was insensitive to Fbxo7 in vitro (Figure 3C); however, cyclin D1/cdk6 levels were sensitive to Fbxo7 levels in vivo (Figure 2E). Additional mechanisms like increasing nuclear concentrations of D cyclin/cdk6 or increasing the half-life of cyclin D/cdk6/p27 proteins are being investigated.
The ability of Fbxo7 to stimulate cyclin D/cdk6 activity is supported by the fact that knockdown of the protein resulted in decreased cyclin D/cdk6 complexes and impaired de novo assembly of viral and D cyclins with cdk6. Conversely, overexpression of Fbxo7 increased cyclin D/cdk6 activity and E2F activation and enhanced the assembly of D cyclins specifically with cdk6. Increasing the activity of known proto-oncogenes suggests that Fbxo7 may itself function as a proto-oncogene. This was tested by showing that its expression transformed NIH3T3 cells, which became invasive and formed tumors in nude mice (Figure 5B and D). Importantly, these phenotypes were reversed by reducing cdk6 levels, supporting the idea that Fbxo7 transformation is due to its effects on cdk6 (Figure 6I and J). Moreover, Fbxo7 levels were highly elevated in human lung and colon cancer biopsies when compared to the normal tissues (Figure 7A and B). The role of Fbxo7 in initiating or maintaining the growth of human tumor cells is currently being investigated, as is its potential use as a prognostic factor. The ubiquitin ligase function of Fbxo7 contributed to, but was not required for, transformation. We speculate that other proteins ubiquitinated by Fbxo7 will be linked in their regulation with cdk6 activity. Thus, Fbxo7 would coordinate ubiquitination of substrates with entry into the cell cycle and act as a platform to relay or partition extracellular signals.
Materials and methods
Cell culture
Human osteosarcoma (U2OS) and murine fibroblast (NIH3T3) cell lines were obtained from Cancer Research UK (LRI Cell Production). p21−/−p27−/− MEFs were obtained from C Sherr and J Roberts. Cells were grown in DMEM supplemented with 10% fetal calf serum (Helena Biosciences), 2 mM glutamine, 100 U/ml penicillin and streptomycin, at 37°C in a humidified 5% CO2 atmosphere. Cells were seeded the day before transfection using Fugene (Cat. #1814443, Roche). NIH3T3-derived cell lines were made by transfection and p21−/−p27−/− cell lines by retroviral infection with Fbxo7 constructs and selection in media containing 600 μg/ml of G418 sulfate (Cat. #11811-064, Gibco), and screened for expression by RT–PCR and Western blot. Anchorage-independent growth was assayed by soft agar assays, as previously described (Radkov et al, 2000). Cell lines were stained with rhodamine-conjugated phalloidin (Molecular Probes), and visualized by confocal fluorescence microscopy. Invasion assays (Cat. #ECM550, Chemicon International) were performed in triplicate as per the manufacturer's protocol. For tumor formation assays, 5 × 106 3T3/vec or 3T3/Fbxo7 cells were injected subcutaneously into 6-week-old athymic nude mice. At 20 days post-infection (p.i.), tumors were noted, and at 30 days p.i., both groups were culled.
DNA and siRNA
The pGAD-Fbxo7 clone contained bp 384–1143 of Fbxo7. The 3′ sequences were amplified from a plasmid, received from J Harper, that contained bp 715–1569. 5′sequences were amplified from a HeLa cDNA library. A full-length Fbxo7 clone was constructed by three-way ligation into pcDNA3 (Invitrogen), modified with either flag or T7 epitope.
dsRNA against Fbxo7 (bp 129–149) and control dsRNA (Cat. #80-11310-A) were purchased from Xeragon, and transfected with Effectene (Qiagen). A hairpin sequence against murine cdk6 (5′-GACTTGACCACTTACTTGG-3′) was cloned into pRS (Brummelkamp et al, 2002). Retroviruses with vector or CDK6-4 were infected into NIH3T3 cell lines with 2 μg/ml puromycin selection to create stable knockdowns of cdk6.
In vivo and in vitro interaction assays
GST fusion proteins were made by cloning cyclin D1, D3, cdk4 and cdk6 in pGEX(KG) (Stratagene). GST-p27 proteins were from D Mann. Proteins were purified on a GST resin. In vitro binding assays were performed with equivalent amounts of GST proteins as previously described (Jimenez et al, 1999). Immunoprecipitations for cell cycle proteins that associated with Fbxo7 were performed according to a method that has been previously described (Diehl et al, 1997), and immunoprecipitations for cyclin D/cdk complexes were as previously described (Mann et al, 1999). In vitro kinase assays with immunoprecipitated cdks, using pRb as a substrate, were performed in triplicate as previously described (Laman et al, 2001). 32P incorporation was quantified using a Cyclone phosphoimager. The following antibodies were used for immunoprecipitation or Western blotting: cdk2 (SC163), cdk4 (SC260), cdk6 (SC177), cyclin D3 (SC182), cyclin A (SC751), PCNA (SC56) and cyclin D1 (SC246) (Santa Cruz Biotechnology Inc.); HA (12CA5) and cyclin D1 (287.4) (RMAS, Cancer Research UK); flag (F3165, Sigma); T7 (Cat. #69522-3, Novagen). Anti-rabbit IgG and anti-mouse IgG antibodies conjugated to horseradish peroxidase were from Santa Cruz Biotechnology Inc. and Jackson ImmunoResearch Laboratories. The polyclonal antibody against the C-terminus of Fbxo7 was made by RMAS, Cancer Research UK. Tissue immunostaining using the Fbxo7 antibody (1/400) on paraffin-embedded sections was performed as previously described (Ye et al, 2000). Multiple tumor tissue array slides were obtained from the Cooperative Human Tissue Network and the Tissue Array Research Program (TARP) of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Gene expression microarrays
Quality and amount of starting RNA was confirmed using an Agilent Bioanalyser. A 10 μg portion of total RNA from 3T3/vec and clonal 3T3/Fbxo7 cell lines was used to generate first-strand cDNA using a T7-oligo(dT) primer. After second-strand synthesis, in vitro transcription was performed with biotinylated UTP and CTP (Enzo Diagnostics), resulting in ∼100-fold amplification. Target cDNAs were processed according to the manufacturer's protocol using an Affymetrix GeneChip Instrument System. Spike controls were added to 20 μg fragmented cRNA before overnight hybridization on mouse 430A arrays. Arrays were stained with streptavidin–phycoerythrin and scanned on an Affymetrix GeneChip scanner. The 3′/5′ ratios for GAPDH were confirmed within acceptable limits, and BioB spike controls were present, with BioC, BioD and CreX present in increasing intensity. RMA log expression units (Irizarry et al, 2003) were calculated from Affymetrix GeneChip array data using the Bioconductor software suite (www.bioconductor.org) for the R statistical programming language (www.r-project.org). Heat maps were generated using Treeview from http://rana.lbl.gov/EisenSoftware.htm. Differences between independent samples were identified by _t_-test, and _P_-values adjusted to take account of multiple testing error using the Benjamini and Yuketieli algorithm (Benjamini and Yekutieli, 2001) implemented within Bioconductor (the multitest package). Significance was accepted at 0.05. Data sets were deposited with MIAMExpress, European Bioinformatics Institute (accession number E-MEXP-369).
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank J Harper, M Pagano, C Sherr and J Roberts for reagents, S Hewitt for tissue microarrays and N Davies, D Bourboulia, E Sanij, S Direkze, D Guiliano and A Godfrey for technical assistance. We thank G Peters, D Mann, T Sharp and D Lagos for comments on the manuscript. This work was funded by Cancer Research UK and HL was funded by the Association for International Cancer Research.
References
- Alt JR, Cleveland JL, Hannink M, Diehl JA (2000) Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev 14: 3102–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt JR, Gladden AB, Diehl JA (2002) p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem 277: 8517–8523 [DOI] [PubMed] [Google Scholar]
- Bagui TK, Mohapatra S, Haura E, Pledger WJ (2003) P27Kip1 and p21Cip1 are not required for the formation of active D cyclin–cdk4 complexes. Mol Cell Biol 23: 7285–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir T, Pagano M (2003) Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv Cancer Res 88: 101–144 [DOI] [PubMed] [Google Scholar]
- Bates S, Peters G (1995) Cyclin D1 as a cellular proto-oncogene. Semin Cancer Biol 6: 73–82 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple hypothesis testing under dependency. Ann Stat 29: 1165–1188 [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2: 243–247 [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M (1999) Identification of a family of human F-box proteins. Curr Biol 9: 1177–1179 [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ (1999) The p21(Cip1) and p27(Kip1) CDK ‘inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18: 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Doglioni C, Yan Z, Lestani M, Menestrina F, Sorio C, Benedetti A, Vinante F, Pizzolo G, Inghirami G (1998) Differential expression of cyclin-dependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am J Pathol 152: 209–217 [PMC free article] [PubMed] [Google Scholar]
- Corcoran MM, Mould SJ, Orchard JA, Ibbotson RE, Chapman RM, Boright AP, Platt C, Tsui LC, Scherer SW, Oscier DG (1999) Dysregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene 18: 6271–6277 [DOI] [PubMed] [Google Scholar]
- Dai K, Kobayashi R, Beach D (1996) Physical interaction of mammalian CDC37 with CDK4. J Biol Chem 271: 22030–22034 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ (1999) SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15: 435–467 [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Sherr CJ (1997) A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol 17: 7362–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Yang W, Rimerman RA, Xiao H, Emili A (2003) Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol Cell Biol 23: 1764–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Zindy F, Sherr CJ (1997) Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin–proteasome pathway. Genes Dev 11: 957–972 [DOI] [PubMed] [Google Scholar]
- Ericson KK, Krull D, Slomiany P, Grossel MJ (2003) Expression of cyclin-dependent kinase 6, but not cyclin-dependent kinase 4, alters morphology of cultured mouse astrocytes. Mol Cancer Res 1: 654–664 [PubMed] [Google Scholar]
- Germain D, Russell A, Thompson A, Hendley J (2000) Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J Biol Chem 275: 12074–12079 [DOI] [PubMed] [Google Scholar]
- Haglund K, Di Fiore PP, Dikic I (2003) Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci 28: 598–603 [DOI] [PubMed] [Google Scholar]
- Hall M, Peters G (1996) Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res 68: 67–108 [DOI] [PubMed] [Google Scholar]
- Hayette S, Tigaud I, Callet-Bauchu E, Ffrench M, Gazzo S, Wahbi K, Callanan M, Felman P, Dumontet C, Magaud JP, Rimokh R (2003) In B-cell chronic lymphocytic leukemias, 7q21 translocations lead to overexpression of the CDK6 gene. Blood 102: 1549–1550 [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19: 141–172 [DOI] [PubMed] [Google Scholar]
- Horak J (2003) The role of ubiquitin in down-regulation and intracellular sorting of membrane proteins: insights from yeast. Biochim Biophys Acta 1614: 139–155 [DOI] [PubMed] [Google Scholar]
- Ilyin GP, Rialland M, Pigeon C, Guguen-Guillouzo C (2000) cDNA cloning and expression analysis of new members of the mammalian F-box protein family. Genomics 67: 40–47 [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK, Eldridge AG (2002) The SCF ubiquitin ligase: an extended look. Mol Cell 9: 923–925 [DOI] [PubMed] [Google Scholar]
- Jimenez G, Verrijzer CP, Ish-Horowicz D (1999) A conserved motif in goosecoid mediates groucho-dependent repression in Drosophila embryos. Mol Cell Biol 19: 2080–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib ZA, Matsushime H, Valentine M, Shapiro DN, Sherr CJ, Look AT (1993) Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas. Cancer Res 53: 5535–5541 [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P (2004) Mouse development and cell proliferation in the absence of D-cyclins. Cell 118: 477–491 [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11: 847–862 [DOI] [PubMed] [Google Scholar]
- Laman H, Coverley D, Krude T, Laskey R, Jones N (2001) Viral cyclin–cyclin-dependent kinase 6 complexes initiate nuclear DNA replication. Mol Cell Biol 21: 624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman H, Mann DJ, Jones NC (2000) Viral-encoded cyclins. Curr Opin Genet Dev 10: 70–74 [DOI] [PubMed] [Google Scholar]
- Mahony D, Parry DA, Lees E (1998) Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene 16: 603–611 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M (2004) Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118: 493–504 [DOI] [PubMed] [Google Scholar]
- Mann DJ, Child ES, Swanton C, Laman H, Jones N (1999) Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J 18: 654–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY (1994) D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol 14: 2066–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F (2004) Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430: 226–231 [DOI] [PubMed] [Google Scholar]
- Matushansky I, Radparvar F, Skoultchi AI (2003) CDK6 blocks differentiation: coupling cell proliferation to the block to differentiation in leukemic cells. Oncogene 22: 4143–4149 [DOI] [PubMed] [Google Scholar]
- McConnell BB, Gregory FJ, Stott FJ, Hara E, Peters G (1999) Induced expression of p16(INK4a) inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin–CDK-inhibitor complexes. Mol Cell Biol 19: 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO (1995) Principles of CDK regulation. Nature 374: 131–134 [DOI] [PubMed] [Google Scholar]
- Pagano M, Benmaamar R (2003) When protein destruction runs amok, malignancy is on the loose. Cancer Cell 4: 251–256 [DOI] [PubMed] [Google Scholar]
- Parry D, Mahony D, Wills K, Lees E (1999) Cyclin D–CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol 19: 1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov SA, Kellam P, Boshoff C (2000) The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma–E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 6: 1121–1127 [DOI] [PubMed] [Google Scholar]
- Schmidt EE, Ichimura K, Reifenberger G, Collins VP (1994) CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res 54: 6321–6324 [PubMed] [Google Scholar]
- Shcherbik N, Haines DS (2004) Ub on the move. J Cell Biochem 93: 11–19 [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1995) D-type cyclins. Trends Biochem Sci 20: 187–190 [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cycles. Science 274: 1672–1677 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512 [DOI] [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW (1996) Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev 10: 1491–1502 [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Martin N, Wilks DP, Tamai K, Huot TJ, Pantoja C, Okumura K, Serrano M, Hara E (2002) Activation of cyclin D1-kinase in murine fibroblasts lacking both p21(Cip1) and p27(Kip1). Oncogene 21: 8067–8074 [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakamura T, Ohtani N, Hampson L, Hampson IN, Shimamoto A, Furuichi Y, Okumura K, Niwa S, Taya Y, Hara E (1999) Regulation of CDK4 activity by a novel CDK4-binding protein, p34(SEI-1). Genes Dev 13: 3027–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N (1997) Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390: 184–187 [DOI] [PubMed] [Google Scholar]
- Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW (1999) A family of mammalian F-box proteins. Curr Biol 9: 1180–1182 [DOI] [PubMed] [Google Scholar]
- Yang J, Kornbluth S (1999) All aboard the cyclin train: subcellular trafficking of cyclins and their CDK partners. Trends Cell Biol 9: 207–210 [DOI] [PubMed] [Google Scholar]
- Ye H, Dogan A, Karran L, Willis TG, Chen L, Wlodarska I, Dyer MJ, Isaacson PG, Du MQ (2000) BCL10 expression in normal and neoplastic lymphoid tissue. Nuclear localization in MALT lymphoma. Am J Pathol 157: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, Kiyokawa H (2002) Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev 16: 2923–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1